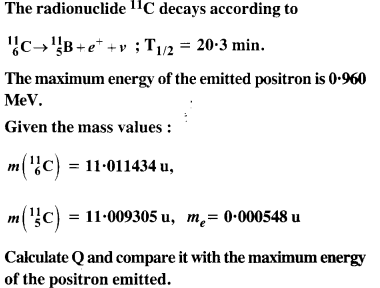
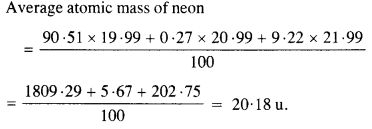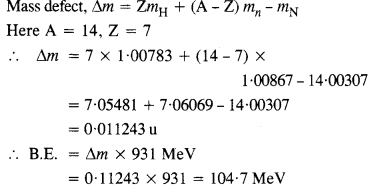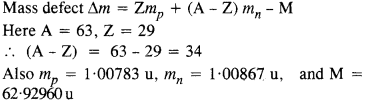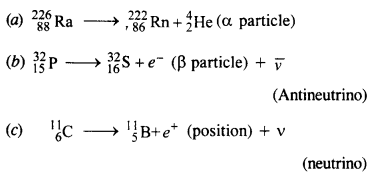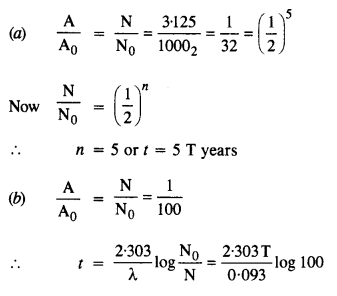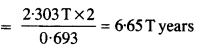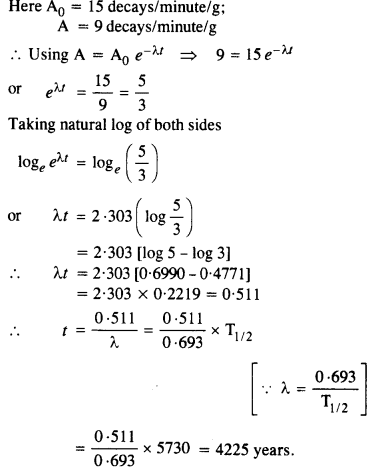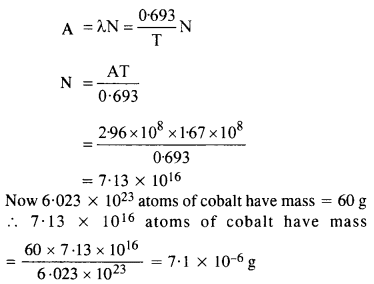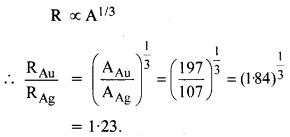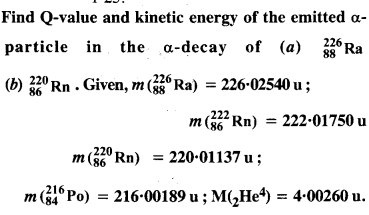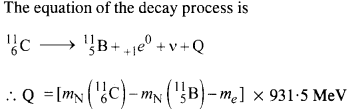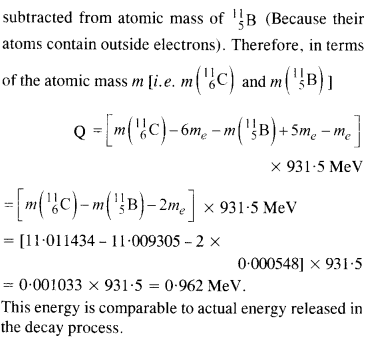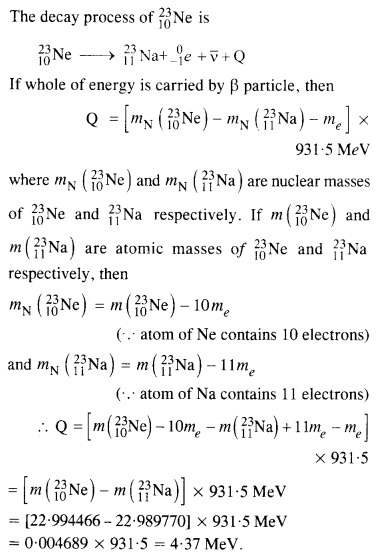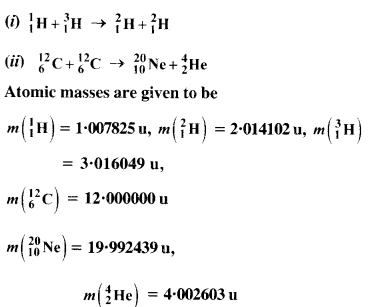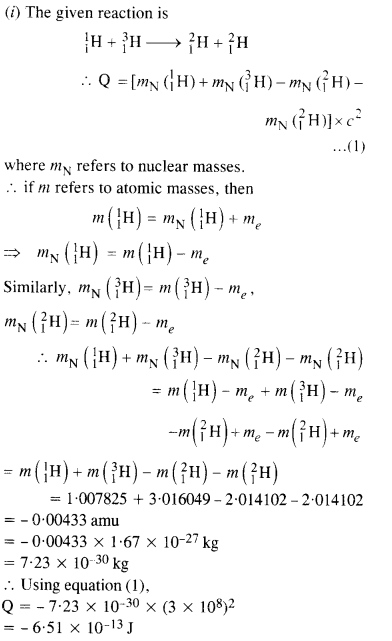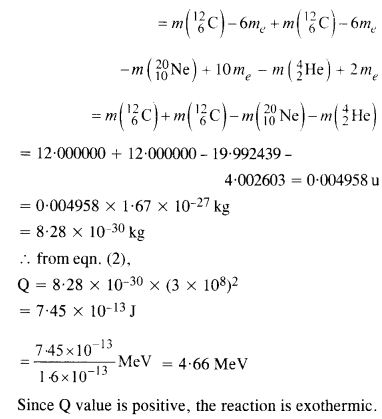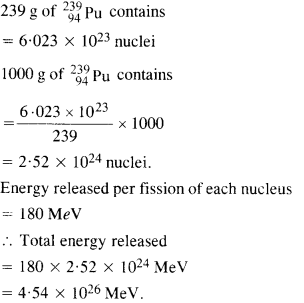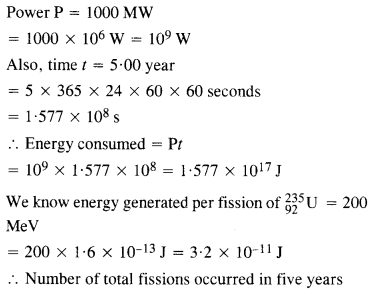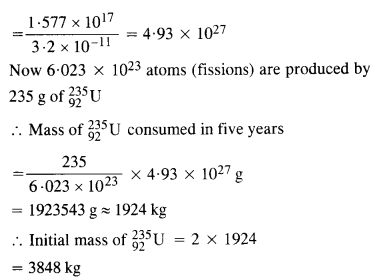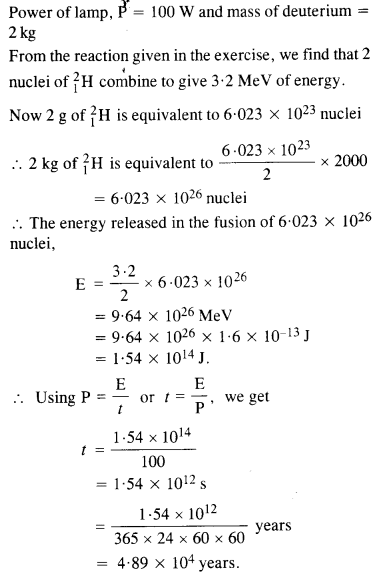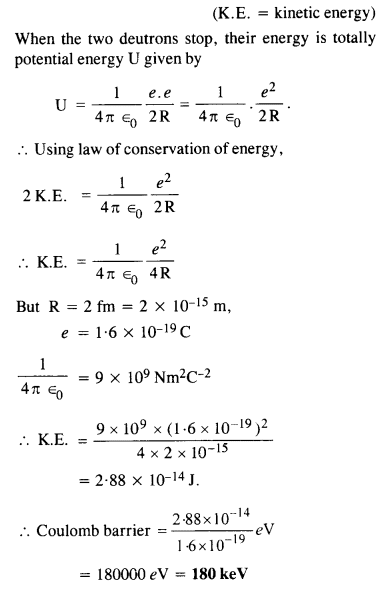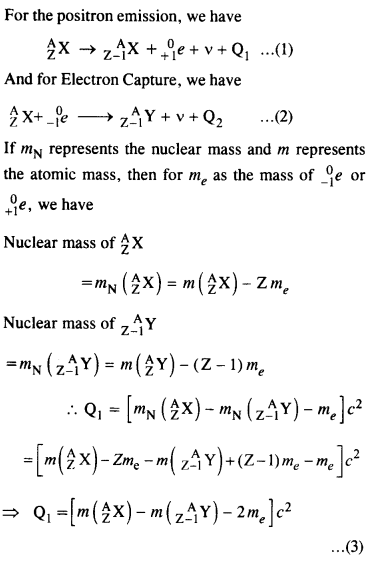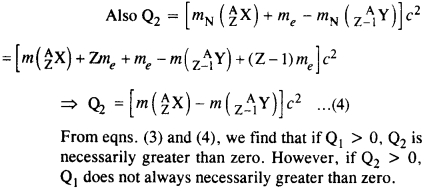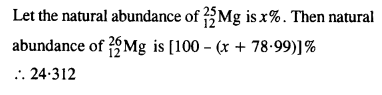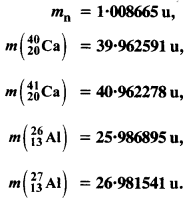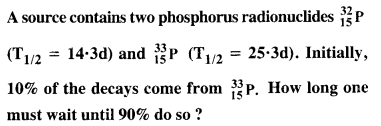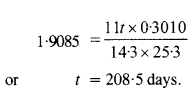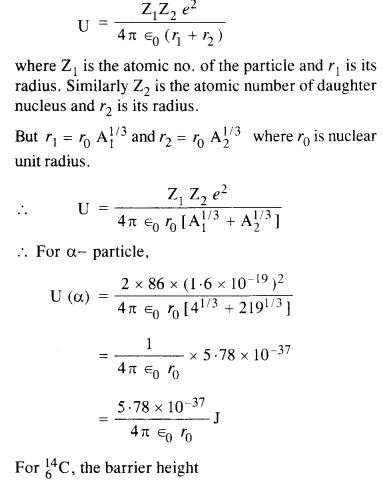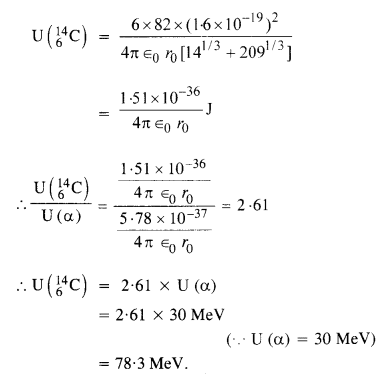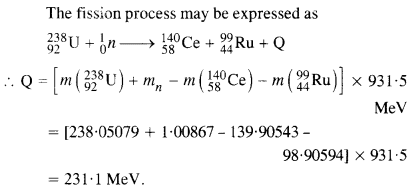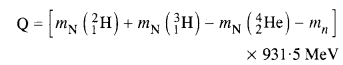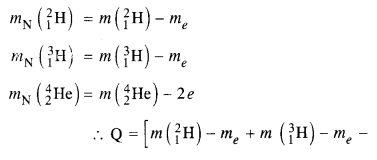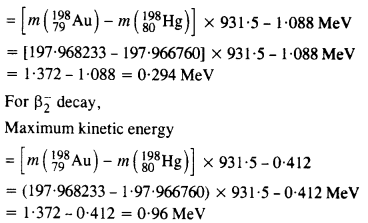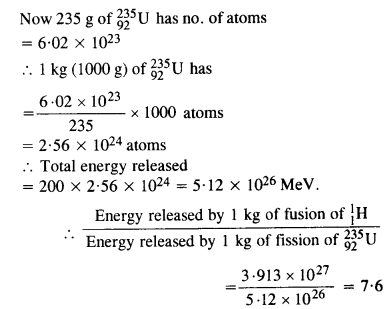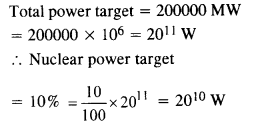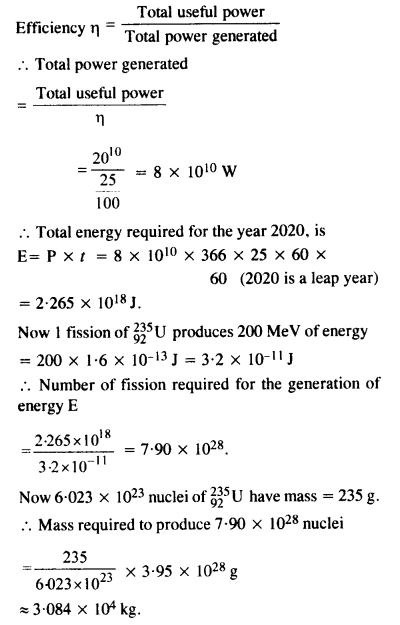Topics and Subtopics in NCERT Solutions for Class 12 Physics Chapter 13 Nuclei:
| Section Name | Topic Name |
| 13 | Nuclei |
| 13.1 | Introduction |
| 13.2 | Atomic Masses and Composition of Nucleus |
| 13.3 | Size of the Nucleus |
| 13.4 | Mass-Energy and Nuclear Binding Energy |
| 13.5 | Nuclear Force |
| 13.6 | Radioactivity |
| 13.7 | Nuclear Energy |
Question 1.
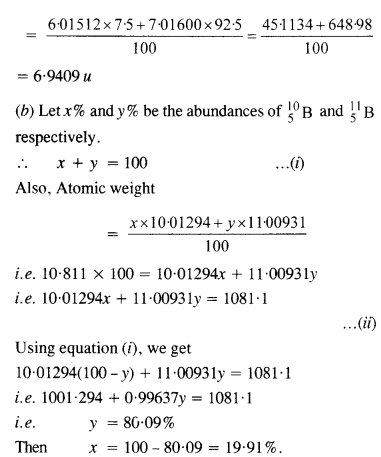
(a) Two stable isotopes of lithium 63Li and 73Li have respective abundance of 7.5% and 92.5%. These isotopes have masses 6.01512 u and 7.01600 u respectively. Find the atomic weight of lithium.
(b) Boron has two stable isotopes 105Li and 115Li .Their respective masses are 10.01294 u and 11.00931 u and the atomic weight of boron is 10.811 u. Find the abundances of 105Li and
115Li
Answer:
(a) Atomic weight of lithium
Question 2.
The three stable isotopes of neon :1020Ne and 1022Ne have respective abundance of 90.51%, 0.27% and 9.22%. The atomic masses of three isotopes are 19.99 u, 20.99 u and 21.99 u, respectively. Obtain the average atomic mass of neon.
Answer:
Question 3.
Obtain the binding energy of a nitrogen nucleus (147N) from the following data :
mH = 1.00783 u
mn = 1.00867 u
mn = 14.00307 u
Give your answer in MeV.
Answer:
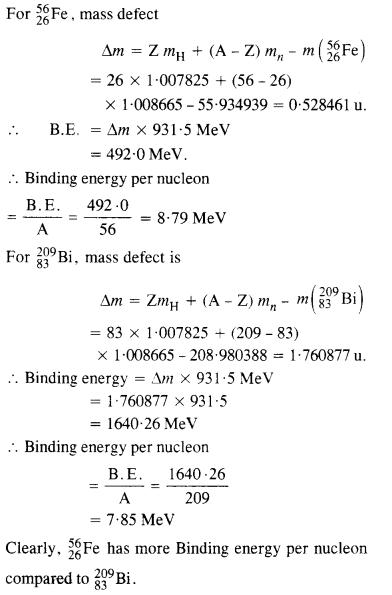
Question 4.
Obtain the binding energy of the nuclei 5626Fe and
in units of 20983Bi from the following data:
mH =1007825u
mn =1008665u
m (5626Fe)= 55.934939 u
m (20983Bi)
Which nucleus has greater binding energy per nucleon ?
Answer:
Question 5.
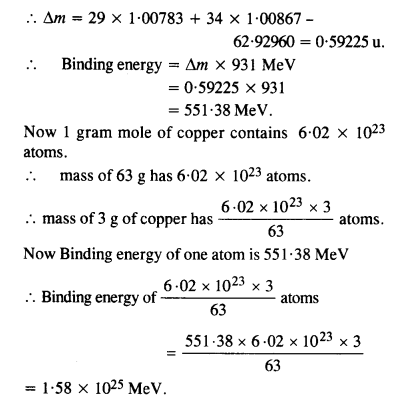
A given coin has a mass of 3.0 g. Calculate the nuclear energy that would be required to separate all the neutrons and protons from each other. For simplicity assume that the coin is entirely made of 6329Cu atoms (of mass 62.92960 u). The masses of proton and neutron are 1.00783 u and 1.00867 u, respectively.
Answer:
Question 6.
Write nuclear equations for :
(a) the α-decay of 22686Ra
(b) the β–-decay of 3215p
(c) the β+-decay of 116p
Answer:
Question 7.
A radioactive isotope has a half-life of T years. After how much time is its activity reduced to 3.125% of its original activity (b) 1% of original value ?
Answer:
Question 8.
The normal activity of living carbon-containing matter is found to be about 15 decays per minute for every gram of carbon. This activity arises from the small proportion of radioactive 146C present with the stable carbon isotope
612C When the organism is dead, its interaction with the atmosphere (which maintains the above equilibrium activity) ceases and its activity begins to drop. From the known half-life (5730 years) of 146C , and the measured activity, the age of the specimen can be approximately estimated. This is the principle of 146C dating used in archaeology. Suppose a specimen from Mohenjodaro gives an activity of 9 decays per minute per gram of carbon. Estimate the approximate age of the Indus-Valley civilisation.
Answer:
Question 9.
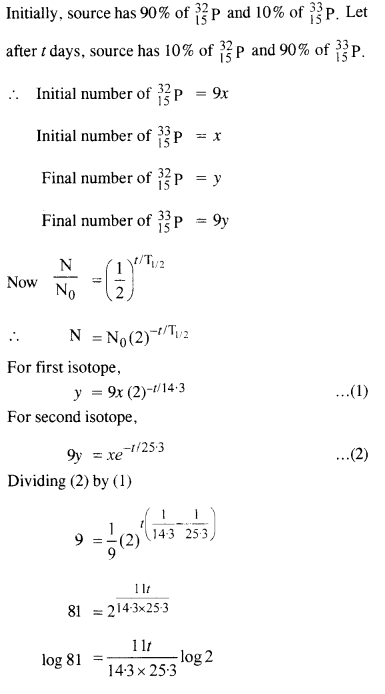
Obtain the amount of 6027Co necessary to provide a radioactive source of 8.0 mCi strength. The half life of 6027Co is 5.3 years.
Answer:
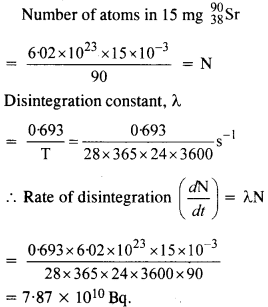
Question 10.
The half life of 9038Sr is 28 years. What is the disintegration rate of 15 mg of this isotope ?
Answer:
Question 11.
Obtain approximately the ratio of the nuclear radii of the gold isotope 19779Au and silver isotope 10747Au.
Answer:
Question 12.
Answer:
Question 13.
Answer:
Here mN stands for the nuclear mass of the element or particle. In order to express the Q value in terms of the atomic masses, 6 me mass has to be subtracted
from atomic mass of 116Au and 5 me mass has to be
Question 14.
The nucleus 2310Ne decays by β~ emission. Write down the p-decay equation and determine the maximum kinetic energy of the electrons emitted. Given that:
m(2310Sr) = 22.994466 u
m(2311Sr) = 22.989770 u.
Answer:
Question 15.
The Q value of a nuclear reaction A + b -> C + d is defined by
[Q = mA + mb-mc– md] c2 where the masses refer to nuclear rest masses. Determine from the given data whether the following reactions are exothermic or endothermic.
Answer:
Question 16.
Suppose, we think of fission of a 5626Fe nucleus into two equal fragments, if 2813Al. Is the fission energetically possible ? Argue by working out Q of the process. Given, m (5626Fe) = 55.93494 u and m (2813Al)= 27.98191
Answer:
Question 17.
The fission properties of 23994Pu are very similar to those of 23592uu. The average energy released per fission is 180 MeV. How much energy, in MeV, is released if all the atoms in 1 kg of pure
23994Pu undergo fission ?
Answer:
Question 18.
A 1000 MW fission reactor consumes half of its fuel in 5.00 y. How much
23592u did it contain initially ? Assume that all the energy generated arises from the fission of 23592u and that this nuclide is consumed by the fission process.
Answer:
Question 19.
How long an electric lamp of 100 W can be kept glowing by fusion of 2.0 kg of deuterium ? The fusion reaction can be taken as
Answer:
Question 20.
Calculate the height of Coulomb barrier for the head on collision of two deuterons. The effective radius of deuteron can be taken to be 2.0 fm.
Answer:
The initial mechanical energy E of the two deutrons before collision is given by
E = 2 K.E.
Question 21.
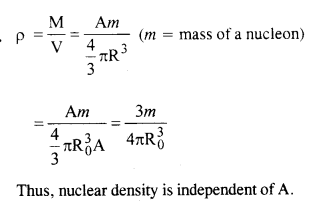
From the relation R = R0 A1/3, where R0 is a constant and A is the mass number of a nucleus, show that nuclear matter density is nearly constant (i.e. independent of A)
Answer:
Question 22.
For the β+ (positron) emission from a nucleus, there is another competing process known as electron capture (electron from an inner orbit, say, the K- shell, is captured by the nucleus and a neutrino is emitted).
Show that if β+ emission is energetically allowed, electron capture is necessarily allowed but not vice-versa
Answer:
Question 23.
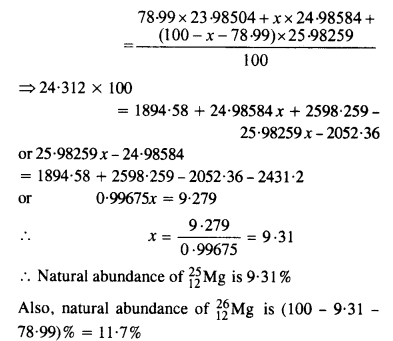
In a Periodic Table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on Earth. The three isotopes and their masses are 2412Mg (23.98504u), ? 2512Mg (24.98584) and 2612Mg (25.98259u). The natural abundance of 2412Mg is 78.99% by mass. Calculate the abundances of the other two isotopes.
Answer:
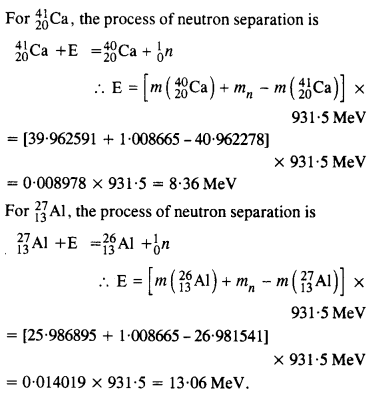
Question 24.
The neutron separation energy is defined as the energy required to remove a neutron from the nucleus. Obtain the neutron separation energies of the nuclei 2412Ca and 2713Al from the following data :
Answer:
Question 25.
Answer:
Question 26.
Under certain circumstances, a nucleus can decay by emitting a particle more massive than an α-particle. Consider the following decay processes :
(a) Calculate the Q values for these decays and determine that both are energetically possible.
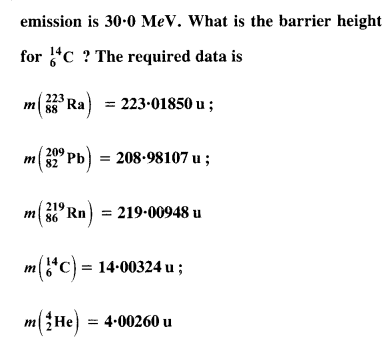
(b) The Coulomb barrier height for α-particle
Answer:
Question 27.
Consider the fission of 23992u by fast neutrons. In one fission event, no neutrons are emitted and the final stable end products, after the beta-decay of the primary fragments, are 14058Ce and 9944Ru. Calculate Q for this fission process. The relevant atomic and particle masses
Answer:
Question 28.
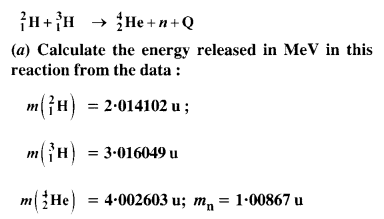
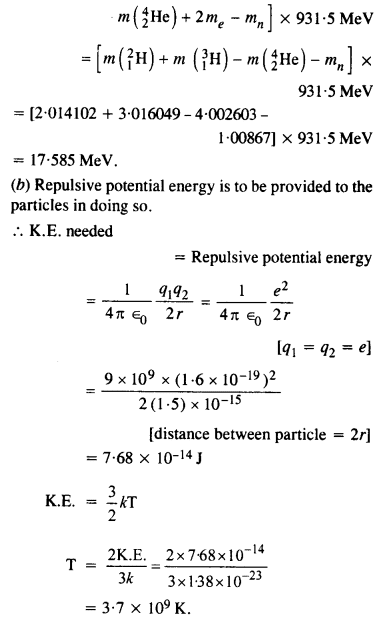
Consider the D-T reaction (deuterium-tritium-fusion) given in eqn. :
(b) Consider the radius of both deuterium and tritium to be approximately 1.5 fm. What is the kinetic energy needed to overcome the Coulomb repulsion ? To what temperature must the gases be heated to inititate the reaction ?
Answer:
From the equation given in the question,
mN refer to nuclear mass of the element given in the brackets and mn = mass of neutron. If in represents the atomic mass, then
Question 29.
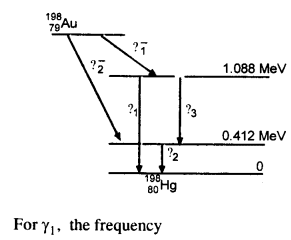
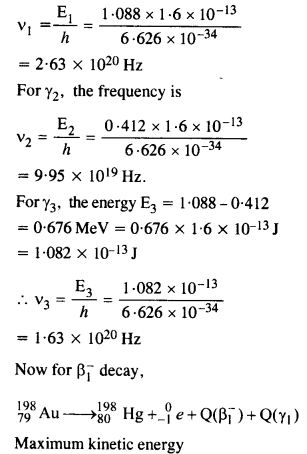
Obtain the maximum kinetic energy of p-particles, and the radiation frequencies to y decays in the following decay scheme. You are given that
m (198Au) = 197.968233 u
m (198Hg) = 197.966760 u
Answer:
The total energy released for the transformation of
19879Au to 19880u can be found by considering the energies of ϒ-rays. We first find the frequencies of the ϒ-rays emitted.
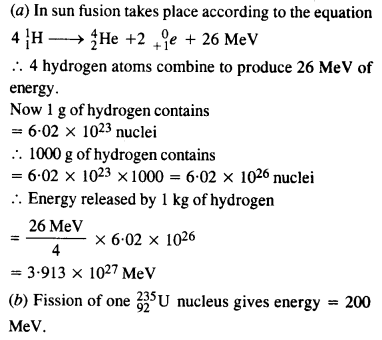
Question 30.
Calculate and compare the energy released by (a) fusion of 1.0 kg of hydrogen deep within the sun and (b) the fission of 1.0 kg of 235U in a fission reactor.
Answer:
Question 31.
Suppose India had a target of producing by 2020 AD, 200,000 MW of electric power, ten percent of which was to be obtained from nuclear power plant. Suppose we are given that, on an average, the efficiency of utilisation (i.e., conversion to electric energy) of thermal energy produced in a reactor was 25%. How much amount of fissionable uranium did our country need per year by 2000 ? Take the heat energy per fission of 235U to be about 200 MeV. Avogadro’s number = 6.023 x 1023 mol-1.
Answer: