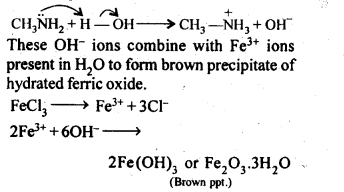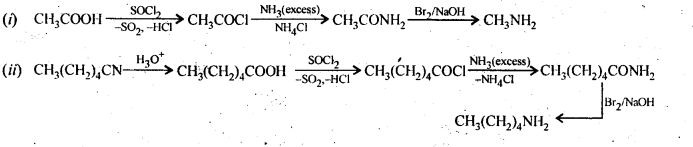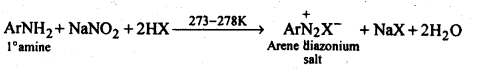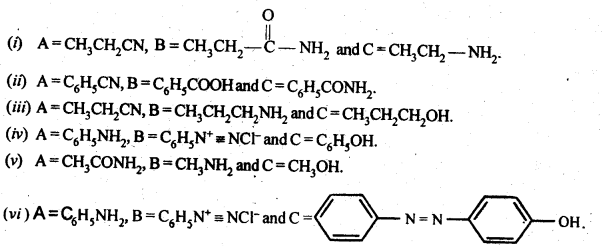Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 13 Amines:
| Section Name | Topic Name |
| 13 | Amines |
| 13.1 | Structure of Amines |
| 13.2 | Classification |
| 13.3 | Nomenclature |
| 13.4 | Preparation of Amines |
| 13.5 | Physical Properties |
| 13.6 | Chemical Reactions |
| 13.7 | Method of Preparation of Diazonium Salts |
| 13.8 | Physical Properties |
| 13.9 | Chemical Reactions |
| 13.10 | Importance of Diazonium Salts in Synthesis of Aromatic Compounds |
NCERT INTEXT QUESTIONS & ANSWERS
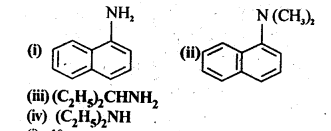
13.1.Classify the following amines as primary, secondary and tertiary:
Ans. (i) 1° (ii)-3° (iii) 1° (iv) 2°
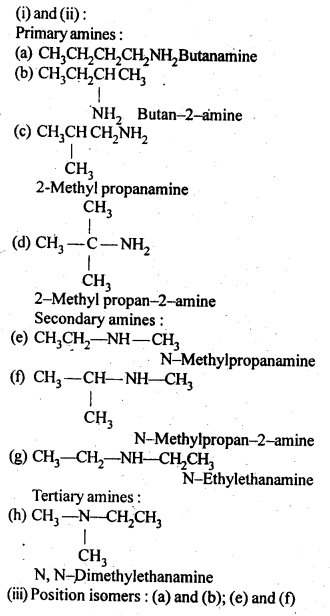
13.2.(i)Write the structures of different isomeric amines corresponding to the molecular formula, C4H11N.
(ii)Write 1UPAC names of all the isomers.
(iii)What type of isomerism is exhibited by different pairs of amines?
Ans.
Chain isomers: (a) and (c); (a) and (d); (b)and(c); (b)and(d)
Metamers: (e) and (g); (f) and (g) Functional isomers: All 10 amines are functional isomers of 2° and 3° amines and vice-versa.
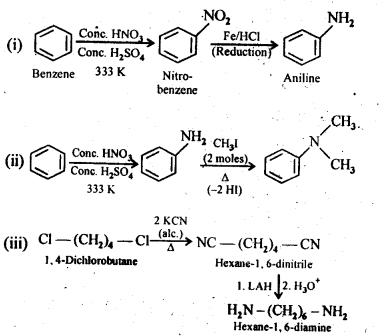
13.3 How will you convert:
(i)Benzene into aniline
(ii)Benzene into N,N-dimethylaniline
(iii)Cl-(CH2)4-Cl into Hexane -1,6- diamine
Ans.
13.4 Arrange the following in increasing order of their basic strength: ‘
(i) C2H5NH2,C6H5NH2,NH3,C6H5CH2NH2 and(C2H5)2 NH
(ii) C2H5NH2,(C2H5)2NH,(C2H5)3N,C6H5NH2
(iii) CH3NH2, (CH3)2NH, (CH3)3N, C6H5NH2, C6H5CH2NH2
Ans.C6H5NH2 < NH3 < C6H5CH2NH2 < C2H5NH2<(C2H5)2NH
(ii)C6H5NH2 < C2H5NH2 < (C2H5)3N <(C2H5)2NH
(iii) C6H5NH2 < C6H5CH2NH2 < (CH3)3 N < CH3NH2<(CH3)2NH
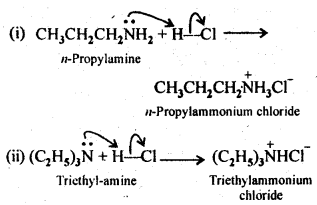
13.5 Complete the following acid-base reactions and name the products:
(i)CH3CH2CH2NH2+HCl ——–>
(ii)(C2H5)3 N+HCl ——–>
Ans.
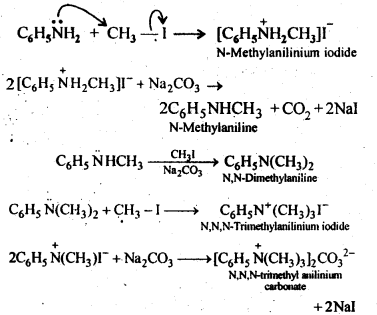
13.6 Write reactions of the final alkylation product of aniline with excess of methyl iodide in the presence of sodium carbonate solution.
Ans.
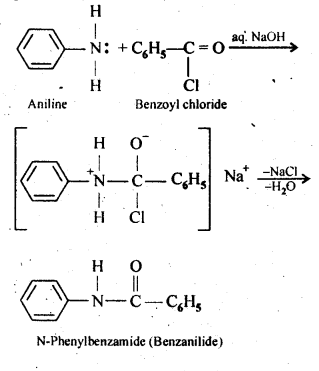
13.7 Write chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
Ans.
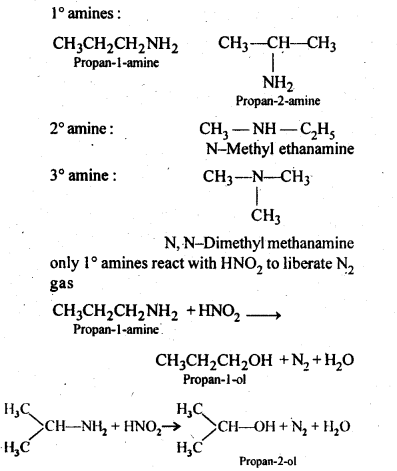
13.8 Write structures of different isomers corresponding to the molecular formula, C3H9N. Write IUPAC names of the isomers which will liberated N2 gas on treatment with nitrons acid.
Ans. In ‘all, four structural isomers are possible. These are:
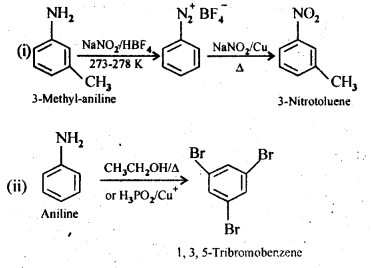
13.9 Convert:
(i)3-Methylanilineinto3-nitrotoluene
(ii)Aniline into 1,3,5- Tribromo benzene
Ans.
NCERT EXRECISES
13.1 Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
(i) (CH3)2 CHNH2 (ii) CH3(CH2)2NH2 (iii) CH3NHCH(CH3)2
(iv) (CH3)3 CNH2 (v) C6H5NHCH3(vi) (CH3CH2)2NCH3
(vii)m-BrC6H4NH2
Ans. (i) Propan-2-amine(1°)
(ii)Propan-1-amine (1°) ,
(iii)N-Methylpropan-2-amine (2°) .
(iv)2-Methylpropan-2-amine(l°)
(v)N-MethylbenzenamineorN-methylaniline(2°)
(vi)N-Ethyl-N-methylethanamine (3°)
(vii)3-Bromobenzenamine or 3-bromoaniline (1°)
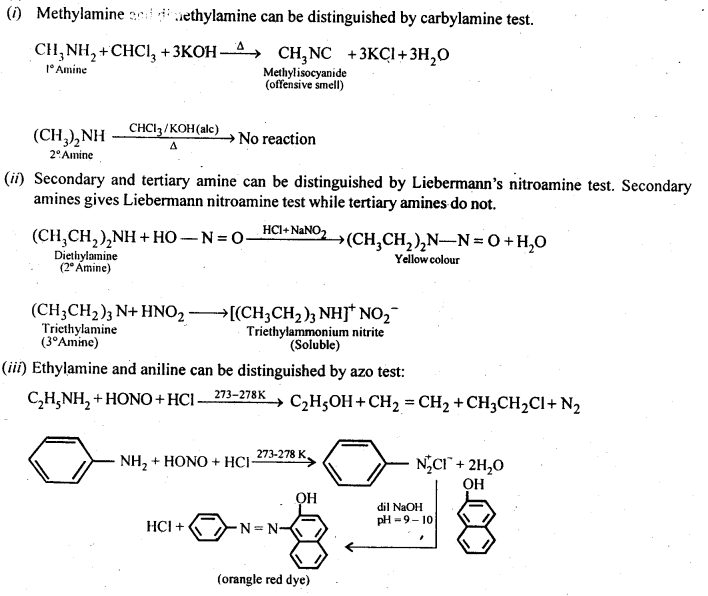
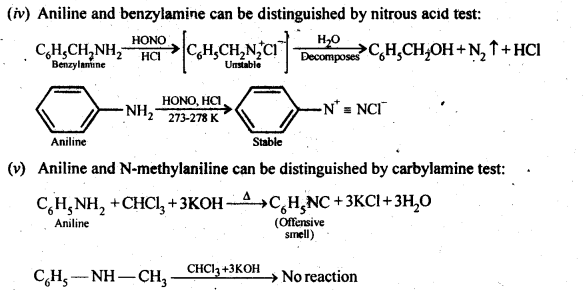
13.2 Give one chemical test to distinguish between the following pairs of compounds:
(i)Methylamine and dimethylamine (ii) Secondary and tertiary amines (iii) Ethylamine and aniline (iv) Aniline and benzylamine (v) Aniline and N-Methylaniline.
Ans.
13.3 Account for the following
(i)pKb of aniline is more than that of methylamine
(ii) Ethylamine is soluble in water whereas aniline is not.
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
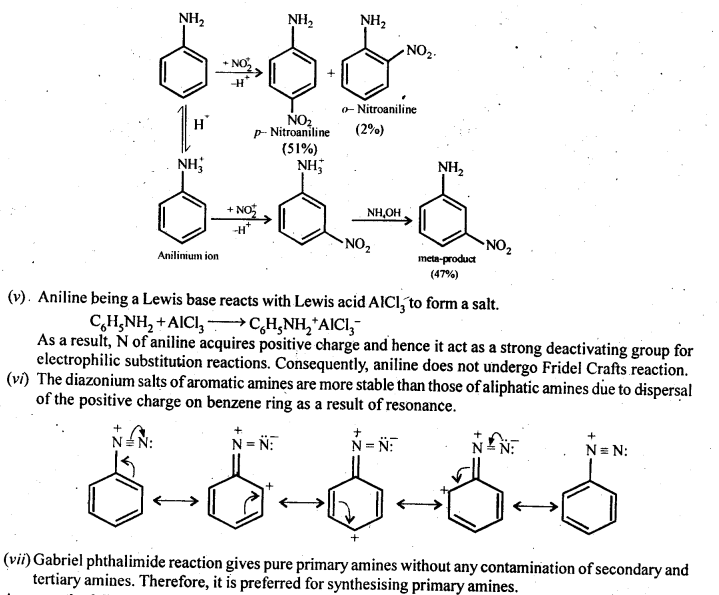
(iv)Although amino group is o and p – directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
(v)Aniline does not undergo Friedel-Crafts reaction.
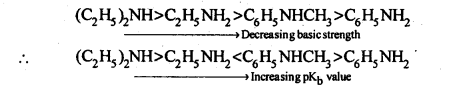
(vi)Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii)Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Ans. (i) In aniline, the lone pair of electrons on the N-atom is delocalised over the benzene ring.
As a result, electron density on the nitrogen . atom decreases. Whereas in CH3NH2,+ I-effect of -CH3 group increases the electron density on the N-atom. Therefore, aniline is a weaker base than methylamine and hence its pKb value is higher than that of methylamine.
(ii) Ethylamine dissolves in water due to intermolecular H-bonding. However, in case of aniline, due to the large hydrophobic part, i.e., hydrocarbon part, the extent of H-bonding is very less therefore aniline is insoluble in water.
(iii)Methylamine being more basic than water, accepts a proton from water liberating OH– ions,
(iv)Nitration is usually carried out with a mixture of cone HNO3 + cone H2SO4. In presence of these acids, most of aniline gets protonated to form ahilinium ion. Therefore, in presence of acids, the reaction mixture consist of aniline and anilinium ion. Now, -NH2 group in aniline is activating and o, p-directing while the -+NH3 group in anilinium ion is deactivating and rw-directing: Nitration of aniline (due to steric hindrance at o-position) mainly gives p-nitroaniline, the nitration of anilinium ion gives m-nitroaniline. In actual practice, approx a 1:1 mixture of p-nitroaniline and m-nitroaniline is obtained. Thus, nitration of aniline gives a substantial amount of m-nitroaniline due to protonation of the amino group.
13.4 Arrange the following:
(i) In decreasing order of pKb values: .
C2H5NH2,C6H5NHCH3,(C2H5)2NH and C6H5NH2
(ii) In increasing order of basic strength:
C6H5NH2, C6H5N(CH3)2, (C2H5)2 NH and CH3NH2.
(iii) In increasing order of basic strength:
(а)Aniline,p-nitroaniline andp-toluidine
(b)C6H5NH2, C6H5NHCH3, C6H5CH2NH2
(iv) In decreasing order of basic strength in gas phase:
C2H5NH2, (C2H5)2NH, (C2H5)3N and NH3
(v) In increasing order of boiling point:
C2H5OH, (CH3)2NH, C2H5NH2
(vi) In increasing order of solubility in water:
C6H5NH2,(C2H5)2NH,C2H5NH2
Ans. (i) Due to delocalisation of lone pair of electrons of the N-atom over the benzene ring,C6H5NH2 and C6H5NHCH3 are far less basic than C2H5NH2 and (C2H,)2NH. Due to +I-effect of the -CH3 group, C6H5NHCH3 is little more basic that C6H5NH2. Among C2H5NH2 and (C2H5)2NH, (C2H5)2NH is more basic than C2H5NH2 due to greater+I-effect of two -C2H5 groups. Therefore correct order of decreasing pKb values is:
(ii) Among CH3NH2 and (C2H5)2NH, primarily due to the greater +I-effect of the two -C2H5 groups over one -CH3 group, (C2H5)2NH is more basic than CH3NH2.In both C6H5NH2 and C6H5N(CH3)2 lone pair of electrons present on N-atom is delocalized over the benzene ring but C6H5N(CH3)2 is more basic due to +1 effect of two-CH3 groups.
(iii) (a) The presence of electron donating -CH3 group increases while the presence of electron withdrawing -NO2 group decreases the basic strength of amines.
(b) In C6H5NH2 and C6H5NHCH3, N is directly attached to the benzene ring. As a result, the lone pair of electrons on the N-atom is delocalised over the benzene ring. Therefore, both C6H5NH2 and C6H5NHCH3 are weaker base in comparison to C6H5CH2NH2. Among C6H5NH2 and C6H5NHCH3, due to +1 effect of-CH3 group C6H5NHCH3 is more basic.
(iv) In gas phase or in non-aqueous solvents such as chlorobenzene etc, the solvation effects i. e., the stabilization of the conjugate acid due to H-bonding are absent. Therefore, basic strength depends only upon the +I-effect of the alkyl groups. The +I-effect increases with increase in number of alkyl groups.Thus correct order of decreasing basic strength in gas phase is,
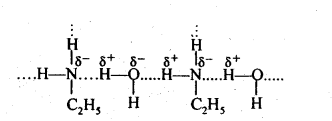
(v)Since the electronegativity of O is higher than thalof N, therefore, alcohols form stronger H-bonds than amines. Also, the extent of H-bonding depends upon flie number of H-atoms on the N-atom, thus the extent of H-bonding is greater in primary amine than secondary amine.
(vi)Solubility decreases with increase in molecular mass of amines due to increase in the size of the hydrophobic hydrocarbon part and with decrease iirthe number of H-atoms on the N-atom which undergo H-bonding.
13.5 How wjll you convert:
(i) Ethanoic acid into methanamine (ii) Hexanenitrile into 1-aminopentane
(iii) Methanol to ethanoic acid. (iv) Ethanamine into methanamine
(v) Ethanoic acid into propanoic acid (vi) Methanamine into ethanamine
(vii) Nitromethane into dimethylamine (viii) Propanoic acid into ethanoic acid?
Ans.
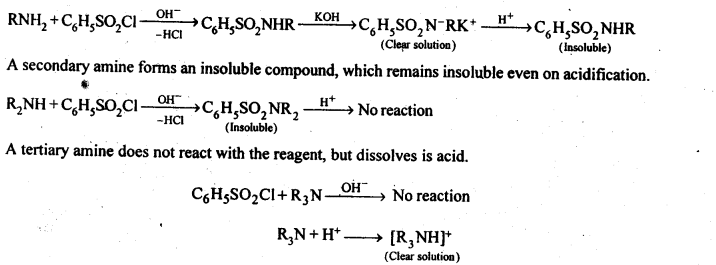
13.6 Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reactions involved.
Ans. The three type of amines can be distinguished by Hinsberg test. In this test, the amine is shaken with benzenesulphonyt chloride (C6H5SO2Cl) in the presence of excess of aqueous NaOH or KOH. A primary amine reacts to give a clear solution, which on acidification yields an insoluble compound.
13.7 Write short notes on the following:
(i) Carbylamine reaction (il) Diazotisation (iii) ‘Hofmann’s bromamide reaction
(iv) Coupling reaction (v) Ammonolysis (vi) Acetylation
(vii) Gabriel phthalimide synthesis
Ans.(i) Carbylamine reaction: Both aliphatic and aromatic primary amines when warmed with chloroform and an alcoholic solution of KOH, produces isocyanides or carbylamines which have very unpleasant odours. This reaction is called carbylamine reaction.
(ii)Diazotisation: The process of conversion of a primary aromatic amino compound into a diazonium salt, is known as diazotisation. This process is carried out by adding an aqueous solution of sodium nitrite to a solution of primary aromatic amine (e.g., aniline) in excess of HCl at a temperature below 5°C.
(iii)Hoffmann’s bromamide reaction: When an amide is treated with bromine in alkali solution, it is converted to a primary amine that has one carbon atom less than the starting amide. This reaction is known as Hoffinann’s bromamide degradation reaction.
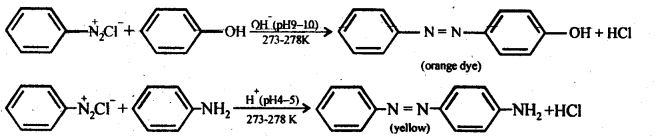
(iv) Coupling reaction: In this reaction, arene diazonium salt reacts with aromatic amino compound (in acidic medium) or a phenol (in alkaline medium) to form brightly coloured azo compounds. The reaction generally takes place at para position to the hydroxy or amino group. If para position is blocked, it occurs at ortho position and if both ortho and para positions are occupied, than no coupling takes place.
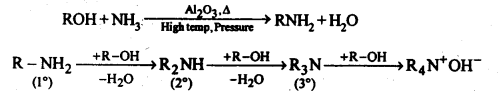
(v) Ammonolysis: It is a process of replacement of either halogen atom in alkyl halides (or aryl halides) or hydroxyl group in alcohols (or phenols) by amino group. The reagent used for ammonolysis is alcoholic ammonia. Generally, a mixture of primary, secondary and tertiary amine is formed.
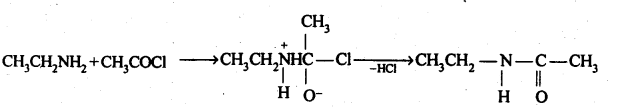
(vi) Acetylation: The process of introducing an acetyl (CH3CO-) group into molecule using acetyl chloride or acetic anhydride is called acetylation.
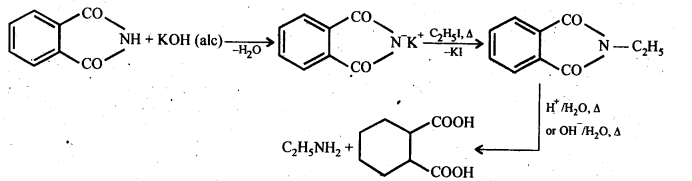
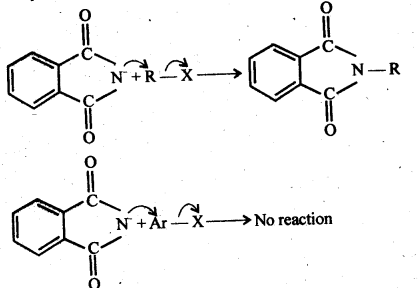
(vii) Gabriel phthalimide synthesis: It is a method of preparation of pure aliphatic and aralkyl primary amines. Phthalimide on treatment with ethanolic KOH gives potassium phathalimide which on heating with a suitable alkyl Or aralkyl halides gives N-substituted phthalimides, which on hydrolysis with dil HCI or with alkali give primary amines.
13.8 Accomplish the following conversions:
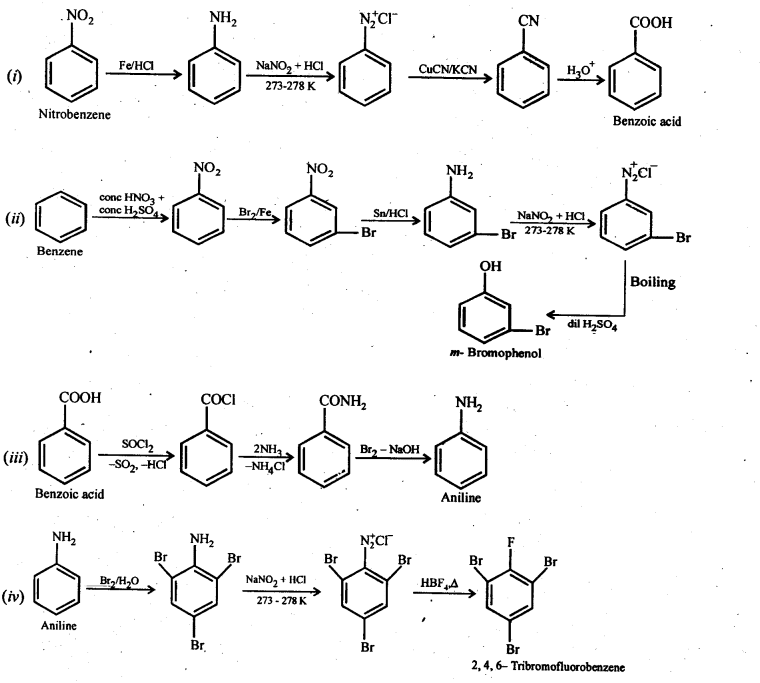
(i) Nitrobenzene to benzoic acid (ii) Benzene to m-bromophenol
(iii) Benzoic acid to aniline (iv) Aniline to 2,4,6-tribromofluorobenzene
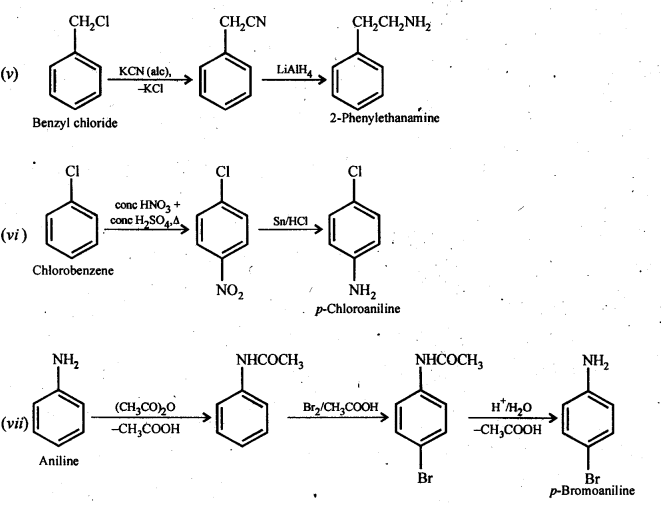
(v) Benzyl chloride to 2-phenylethanamine (vi) Chlorobenzene to p-Chloroaniline
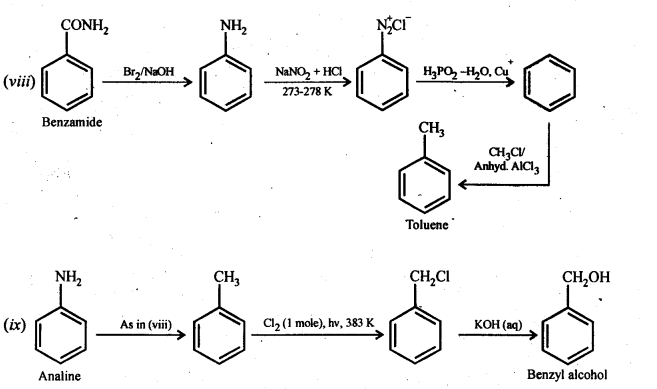
(vii) Aniline to p-bromoaniIine (viii)Benzamide to toluene
(ix) Aniline to benzyl alcohol.
Ans.
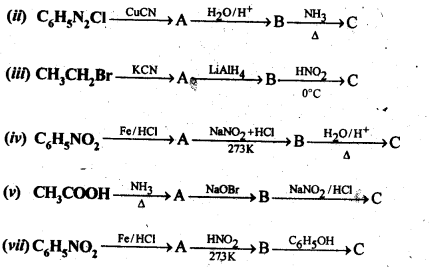
13.9 Give the structures of A,B and C in the following reaction:
Ans.
13.10 An aromatic compound ‘A’on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2 and KOH forms a compound ‘C’ of molecular formula C6H7N. Write the structures and IUPAC names of compounds A,B and C.
Ans. Since the compound ‘C’ with molecular formula C6H7N is formed from compound ‘B’ on treatment with Br2 KOH, therefore, compound ‘B’ must be an amide and ‘C’ must be an amine.
The only amine having the molecular formula C6H7N, i. e., C6H5NH2 is aniline.
Since ‘C’ is aniline, therefore, die amide from which it is formed must be benzamide (C6H5CONH2). Thus, compound‘B’is benzamide. Since compound ‘B’ is formed from compound ‘A’ with aqueous ammonia and heating, therefore, compound ‘A’ must be benzoic acid.
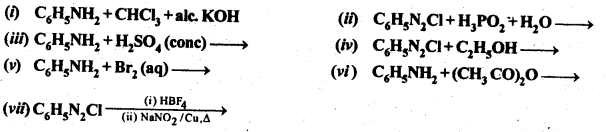
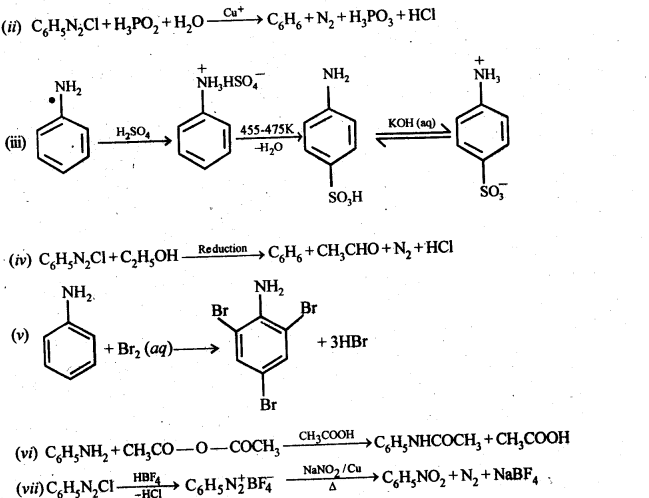
13.11 Complete the following reactions:
Ans.
13.12 Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Ans. The success of Gabriel phthalimide reaction depends upon the nucleophilic attack by the phthalimide anion on the organic halogen compound.
Since aryl halides do not undergo nucleophilic substitution reactions easily, therefore, arylamines, i.e., aromatic, primary amines cannot be prepared by Gabriel phthalimide reaction.
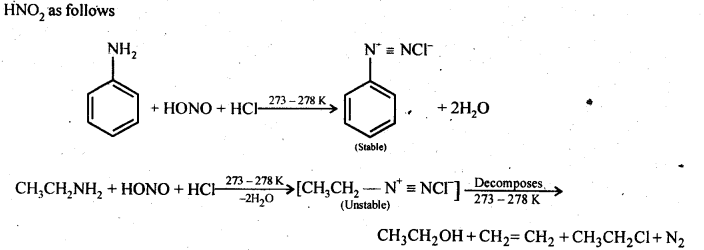
13.13 Write the reactions of (i) aromatic and (ii) aliphatic primary amines with nitrous acid.
Ans. Both aromatic and aliphatic primary amines react with HNO2 at 273-278 K to form aromatic and aliphatic diazonium salts respectively. But aliphatic diazonium salts are unstable even at this low temperature and thus decompose readily to form a mixture of compounds. Aromatic and aliphatic primary amines react with
13.14 Give plausible explanation for each of the following:
(i) Why are amines less acidic than alcohols of comparable molecular masses?
(ii) Why do primary amines have higher boiling point than tertiary amines?
(iii) Why are aliphatic amines stronger bases than aromatic amines?
Ans. (i) Loss of proton from an amine gives an amide ion while loss of a proton from alcohol give an alkoxide ion.
R—NH2—>R—NH– +H+
R—O —H—>R— O– +H+ .
Since O is more electronegative than N, so it wijl attract positive species more strongly in comparison to N. Thus, RO~ is more stable than RNH®. Thus, alcohols are more acidic than amines. Conversely, amines are less acidic than alcohols.
(ii) Due to the presence of two H-atoms on N-atom of primary amines, they undergo extensive intermolecular H-bonding while tertiary amines due to the absence of H-atom on the N-atom do not undergo H-bonding. As a result, primary amines have higher boiling points than tertiary amines of comparable molecular mass.
(iii) Aromatic amines are far less basic than ammonia and aliphatic amines because of following reasons:
(a)Due to resonance in aniline and other aromatic amines, the lone pair of electrons on the nitrogen atom gets delocalised over the benzene ring and thus it is less easily available for protonation. Therefore, aromatic amines are weaker bases than ammonia and aliphatic amines.
(b)Aromatic amines arS more stable than corresponding protonated ion; Hence, they hag very less tendency to combine with a proton to form corresponding protonated ion, and thus they are less basic.