Quick revision notes on the topic Photo-eletric effect of the very important unit “Dual Nature of Matter and Radiation” from the numericals point of view.
After the discovery of X-rays and electron in 1895 and 1897 respectively gave important milestones to the theory of atomic structure. J. J. Thomson applied the concept of mutually perpendicular electric and magnetic field across the discharge tube, was able to determine the speed and the specific charge [charge to mass ratio (e/m)] of the cathode rays.
Electron Emission:
The phenomenon of emission of electrons from the surface of any metal is called as electron emission. There are different ways in which electron emission can take:-
(i) Photoelectric emission
(ii) Thermionic emission
(iii) Field emission emission
(iv) Secondary emission emission
(i) Photoelectric emission
(ii) Thermionic emission
(iii) Field emission emission
(iv) Secondary emission emission
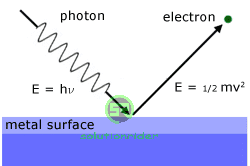
Photons :
The photons are the quanta/packets of light, which are emitted by a source of radiation.
(i) The energy of a photon is given as, E = hv, where v is the frequency of light.
(ii) The momentum of a photon is given as p = hv/c = h/λ
(iii) The rest mass of a photon is zero.
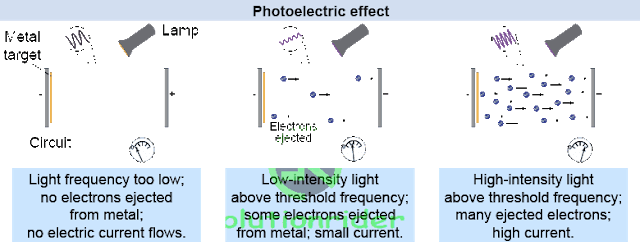
Photo-electric Effect:
Whenever light of any particular frequency is incident on any metal surface, then the emission of electrons is called Photo-electric effect.
The important terms related to photoelectric effect are:
(i) Work function: The minimum energy that is required to eject an electron from the metal is called Work-function of metal. It is denoted as φ.
(ii) Threshold wavelength: The maximum of light that can eject an electron from the metal is called Threshold wavelength of metal. It is denoted as λMAX.
(iii) Threshold frequency: The minimum frequency of light that can eject an electron from the metal surface is called Threshold frequency of metal. It is denoted as v.
Note: The relationship between the work function, threshold wavelength, and the threshold frequency is:
(ii) Threshold wavelength: The maximum of light that can eject an electron from the metal is called Threshold wavelength of metal. It is denoted as λMAX.
(iii) Threshold frequency: The minimum frequency of light that can eject an electron from the metal surface is called Threshold frequency of metal. It is denoted as v.
Note: The relationship between the work function, threshold wavelength, and the threshold frequency is:
φ = hc /λMAX = hv
Einstein’s Photoelectric Equation
The maximum kinetic energy of photo-electrons, is given as:
(Ek)max = hv – φ = h(v –v )
where, v is threshold frequency,
v is frequency of incident light.
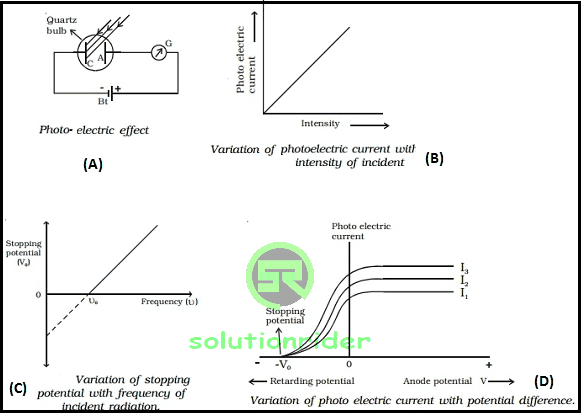
Stopping Potential
The minimum negative potential that is given to anode plate, at which photoelectric current becomes zero is called stopping potential (Vo). The stopping potential is related to the maximum kinetic energy of the emitted photo-electrons as:
(Ek)max = 1/2 mv2max = eV
Laws of Photo-eletric effect:
1) The rate of emission of photo-electrons also called as photo-electric current is is directly proportional to the intensity of incident light.
2) Below threshold frequency, emission of photo electrons cannot take place.
3) Above threshold frequency, the maximum kinetic energy of the emitted photo-electrons depends upon the frequency of incident light.