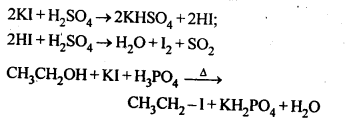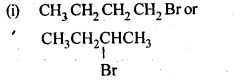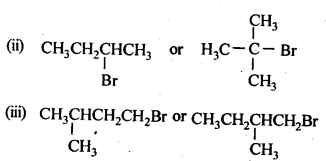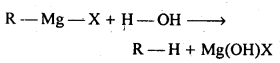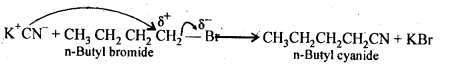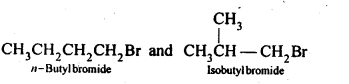Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes:
| Section Name | Topic Name |
| 10 | Haloalkanes and Haloarenes |
| 10.1 | Classification |
| 10.2 | Nomenclature |
| 10.3 | Nature of C–X Bond |
| 10.4 | Methods of Preparation of Haloalkanes |
| 10.5 | Preparation of Haloarenes |
| 10.6 | Physical Properties |
| 10.7 | Chemical Reactions |
NCERT IN TEXT QUESTIONS
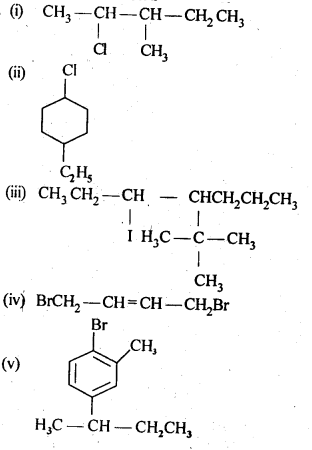
10.1 Write structures of the following compounds:
(i)2-Chloro-3-methylpentane
(ii)1-Chloro-4-ethylcydohexane
(iii)4-tert. Butyl-3-iodoheptane
(iv)1,4-Dibromobut-2-ene
(v)1-Bromo-4-sec. butyl-2-methylbenzene.
Ans.
10.2.Why is sulphuric acid not used during the reaction of alcohols with KI?
Ans. H2SO4 is an oxidising agent. It oxidises HI produced during the reaction to I2 and thus prevents the reaction between an alcohol and HI to form alkyl iodide. To prevent this, a non¬oxidising acid like H3PO3 is used.
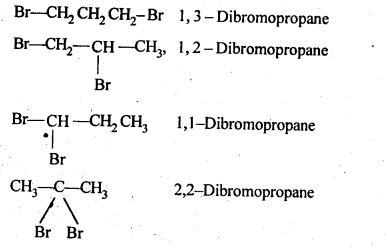
10.3.Write structures of different dihalogen derivatives of propane.
Ans. Four isomers are possible. These are :
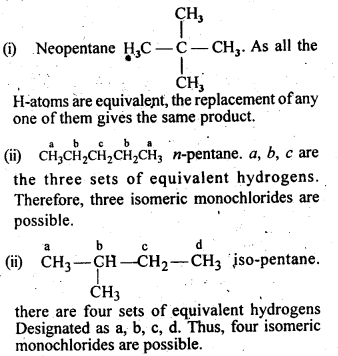
10.4.Among the isomeric alkanes of mdlecular formula C5H12, identify the one that on photochemical chlorination yields
(i)A single monochloride.
(ii)Three isomeric monochlorides.
(iii)Four isomeric monochlorides.
Ans.
10.5 Draw the structures of major monohalo products in each of the following reactions:
Ans.
10.6 Arrange each set of compounds in order of increasing boiling points.
(i)Bromomethane, Bromoform, Chloromethane, Dibromomethane.
(ii)1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
Ans. (i) Chloromethane < Bromomethane <
Dibromomethane < Bromoform
The reason is:
(a)for same alkyl group, B.Pt increases with size of halogen atom.
(b)B.Pt increases as number of halogen atoms increase.
(ii)Isopropyl chloride < 1 – Chloropropane < 1 – Chlorobutane
Reason :
(a)For same halogen, B.Pt. increases as size of alkyl group increases.
(b)B.Pt. decreases as branching increases.
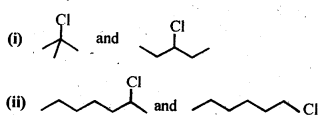
10.7. Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
Ans. In SN2 mechanism, reactivity depends upon the steric hindrance around the C-atom carrying the halogen. Lesser the steric hindrance, faster the reaction.
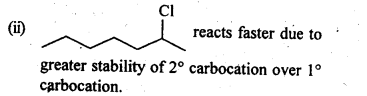
10.8. In the following pairs of halogen compounds, which compound undergoes faster SN1 reaction?
Ans.
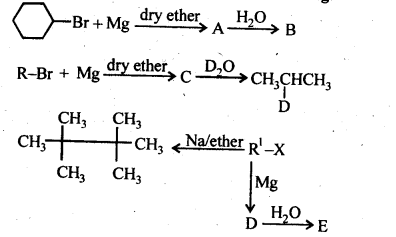
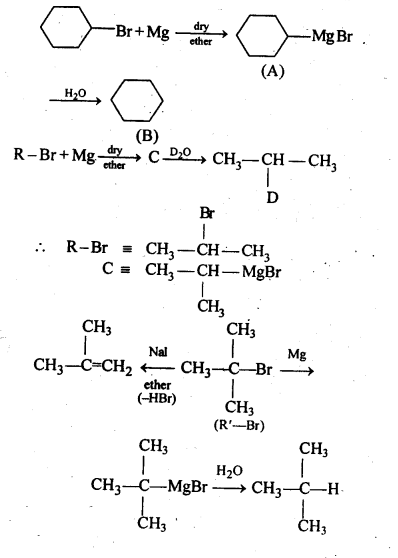
10.9. Identify A, B, C, D, E, R and R1 in the following:
Ans.
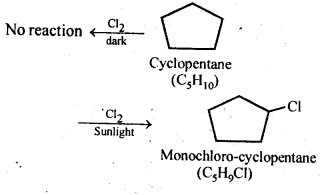
10.10. A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
Ans. The hydrocarbon with molecular formula C5H10 can either a cycloalkane or an alkene.Since the compound does not react with Cl2 in the dark, therefore it cannot be an alkene but must be a cycloalkane. Since the cycloalkane reacts with Cl2 in the presence of bright sunlight to give a single monochloro compound, C5H9Cl, therefore, all the ten hydrogen atoms of the cycloalkanes must be equivalent. Thus, the cycloalkane is cyclopentane.
NCERT EXRECISES
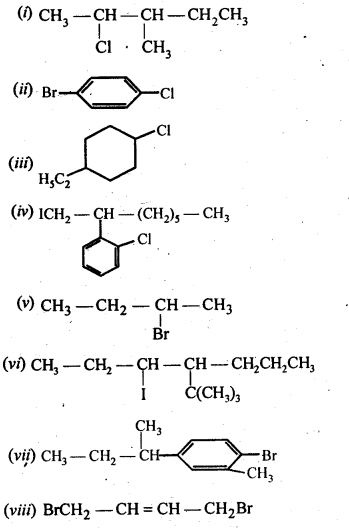
10.1 Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
(i)(CH3)2CHCH(Cl)CH3
(ii) CH3CH2CH(CH3)CH(C2H5)CI
(iii) CH3CH2C(CH3)2CH2I
(iv)(CH3)3CCH2CH(Br)C6H5
(v)CH3CH(CH3)CH(Br)CH3
(vi)CH3C(C2H5)2CH2Br
(vii)CH3C(Cl)(C2H5)CH2CH3
(viii)CH3CH=C(CI)CH2CH(CH3)2
(ix)CH3CH=CHC(Br)(CH3)2
(x)P-CIC6H4CH2CH(CH3)2
(xi)m-ClCH2C6H4CH2C(CH3)3
(xii)o-Br -C6H4CH (CH3)CH2CH3
Ans. (i) 2-Chloro-3methylbutane, 2° alkyl halide
(ii) 3-Chloro-4methyl hexane, 2° alkyl halide
(iii) 1 -Iodo-2,2-dimethylbutane, 1 ° alkyl halide
(iv) l-Bromo-3, 3-dimethyl -1-phenylbutane, 2° benzylic halide
(v) 2-Bromo-3-methylbutane, 2° alkyl halide
(vi) 1-Bromo-2-ethyI-2-methylbutane, 1° alkyl halide
(vii)3-Chloro-3-methylpentane, 3° alkyl halide
(viii) 3-Chloro-5-methylhex-2-ene, vinylic halide
(ix)4-Bromo-4-methylpent-2-ene, allylic halide
(x)1-Chloro-4-(2-methylpropyl) benzene, aryl halide
(xi)1-Chloromethyl-3- (2,2-dimethylpropyl) benzene, 1 ° benzylic halide.
(xii)1-Bromo-2-(l-methylpropyl) benzene,aryl halide.
10.2 Give the IUPAC names of the following compounds:
(i) CH3CH(CI)CH (Br)CH3 (ii) CHF2CBrCIF (iii) CICH2C=CCH2Br (iv) (CCl3)3CCl
(v)CH3C(p-ClC6H4)2CH(Br)CH3 (vi)(CH3)3CCH=C(CI)C6H4I -p
Ans. (i) 2-Bromo-3-chlorobutane
(ii) 1 JBromo-1 -chloro-1,2,2-trifluoroethane
(iii) l-Bromo-4-chlorobut-2-yne
(iv)2-(Trichloromethyl)-l, 1,1,2,3,3,3- heptachloropropane
(v)2-Bromo-3,3-bis-(4-chlorophenyl) butane
(vi)l-Chloro-l-(4-iodophenyl)-3,3- dimethylbut-l-ene.
10.3 Write the structures of the following organic halogen compounds:
(i)2-ChIoro-3-methylpentane
(ii)p-Bromochlorobenzene
(iii)l-Chloro-4-ethylcyclohexane
(iv)2r (2-Chlorophenyl) -1- iodooctane
(v)2-Bromobutane
(vi)4-tert-Butyl-3-iodoheptane
(vii)1-Bromo-4-sec-butyl-2-methylbenzene
(viii)1,4-Dibromobut-2-ene
Ans.
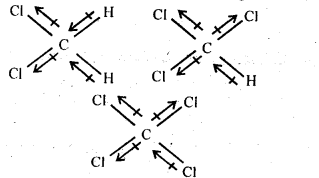
10.4 Which one of the following has the highest dipole moment?
(i)CH3CI2 (ii) CHCl3 (iii) CCI4
Ans. The three dimensional structures of the three compounds along with the direction of dipole moment in each of their bonds are given below:
CCl4 being symmetrical has zero dipole moment. In CHCl3, the resultant of two C – Cl dipole moments is opposed by the resultant of C – H and C – Cl bonds. Since the dipole moment of latter resultant is expected to be smaller than the former, CHCl3 has a finite dipole (1.03 D) moment.
In CH2CI2, the resultant of two C – Cl dipole moments is reinforced by resultant of two C – H dipoles, therefore, CH2CI2 (1 .62 D) has a dipole moment higher than that of CHCl3. Thus, CH2CI2 has highest dipole moment.
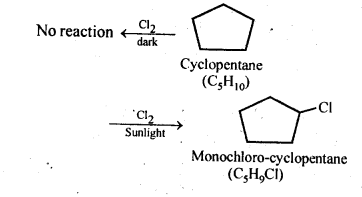
10.5 A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9CI in bright sunlight. Identify the hydrocarbon.
Ans. The hydrocarbon with molecular formula C5H, 0 can either a cycloalkane or an alkene.
Since the compound does not react with Cl2 in the dark, therefore it cannot be an alkene but must be a cycloalkane. Since the cycloalkane reacts with Cl2 in the presence of bright sunlight to give a single monochloro compound, C5H9Cl, therefore, all the ten hydrogen atoms of the cycloalkanes must be equivalent. Thus, the cycloalkane is cyclopentane.
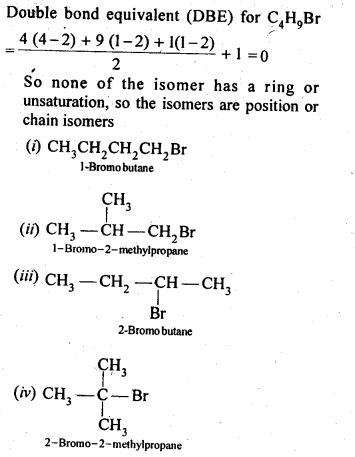
10.6 Write the isomers of the compound having formula C4H9Br.
Ans.
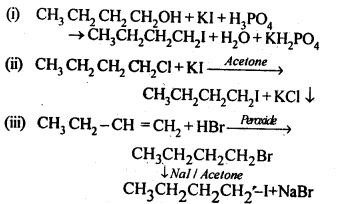
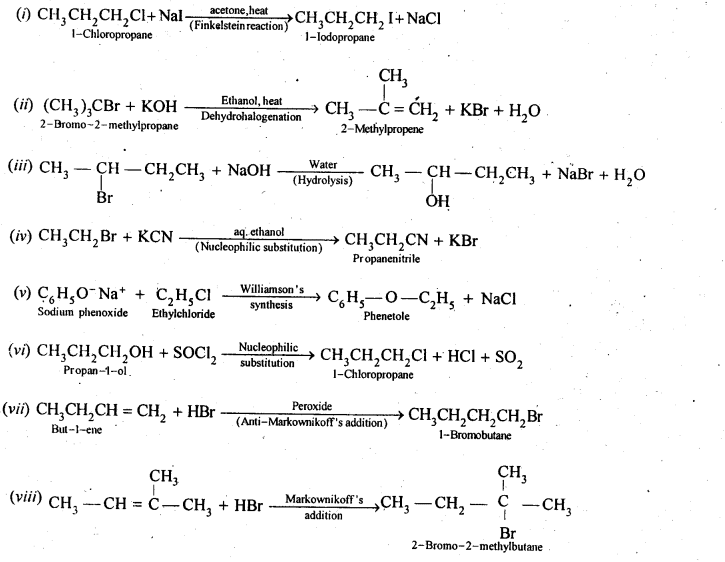
10.7 Write the equations for the preparation of 1-iodoobutanefrom (i)1-butanol (ii)1-chlorobutane (iii) but-l-ene.
Ans.
10.8 What are ambident nucleophiles ? Explain with an example.
Ans. Nucleophiles which can attack through two different sites are called ambident nucleophiles. For example, cyanide ion is a resonance hybrid of the following two structures:
It can attack through carbon to form cyanide and through N to form is O cyanide.
10.9 Which compound in each of the following-pairs . will react faster in SN2 reaction with -OH? (i)CH3Br or CH3I
(ii)(CH3)3CCl or CH3Cl
Ans.(i)Since I– ion is a better leaving group than Br- ion, therefore, CH3I reacts faster CH3Br in SN2 reaction with OH– ion.
(ii)On steric grounds, 1° alkyl halides are more reactive than tert-alkyl halides in SN2 reactions. Therefore, CH3CI will react at a faster rate than (CH3)3CCl in a SN2 reaction with OH– ion.
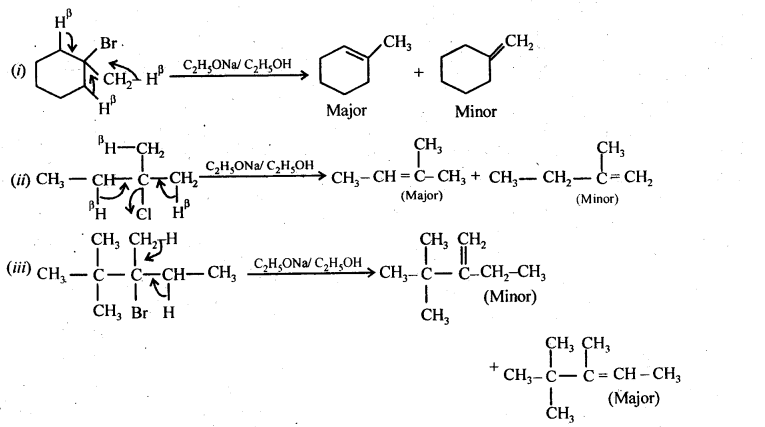
10.10 Predict all the alkenes that would be formed by dehydrohalogenationof the following halides with sodium ethoxide in ethdnol and identify the major alkene:
(i)1-Bromo-l-methylcyclohexane
(ii)2-Chloro-2-methylbutane.
(ill) 2,2,3-Trimethyl-3-bromopentane.
Ans.
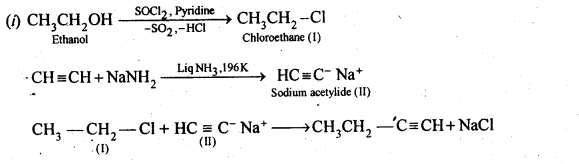
10.11 How will you bring about the following conversions?
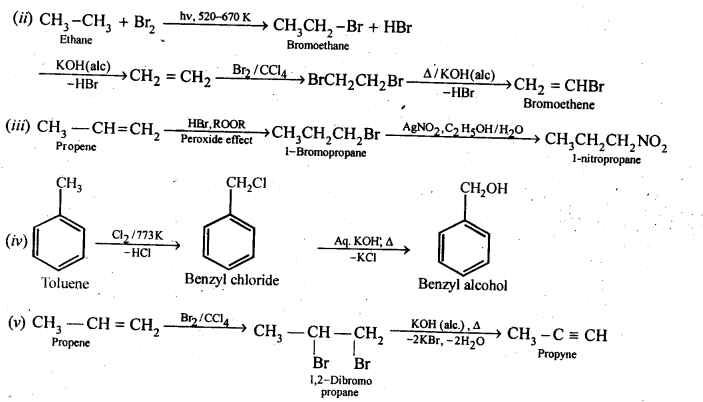
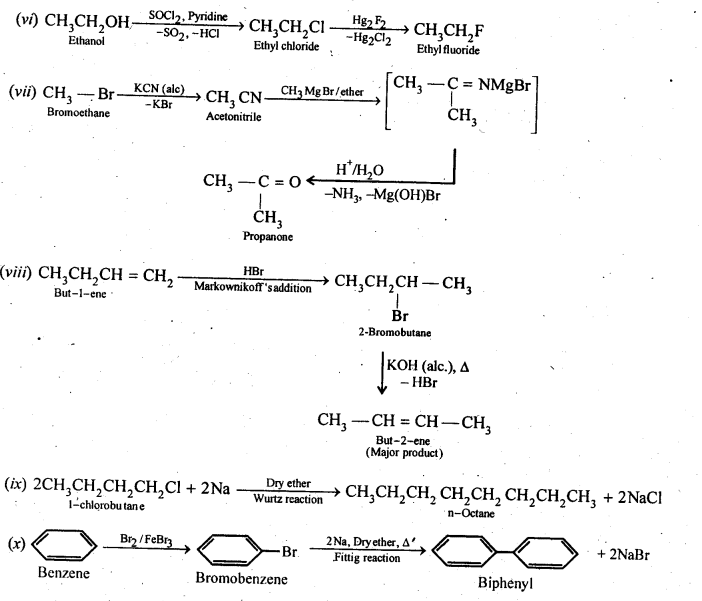
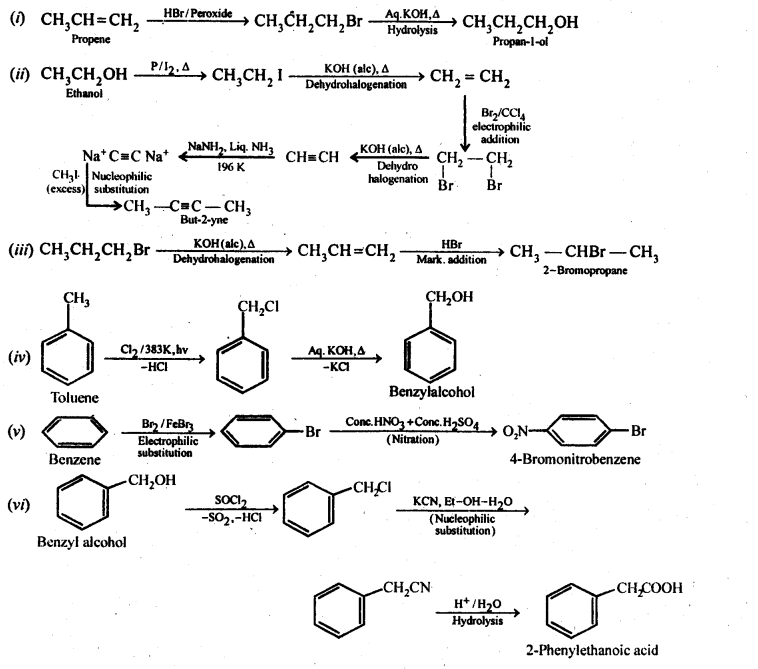
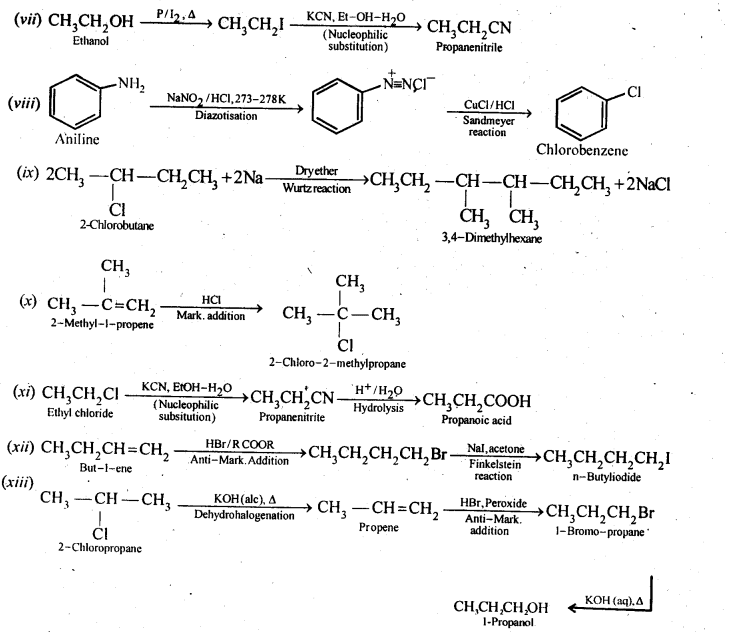
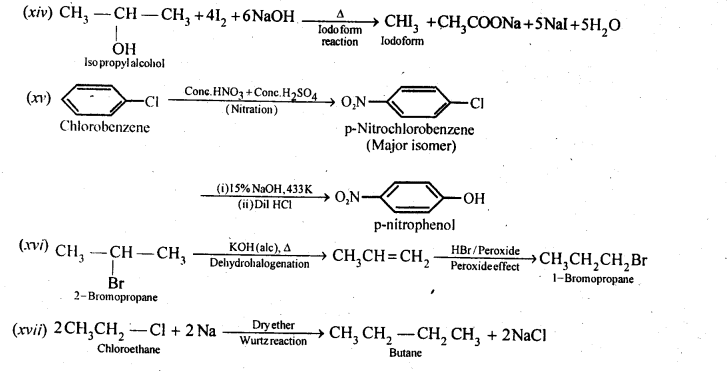
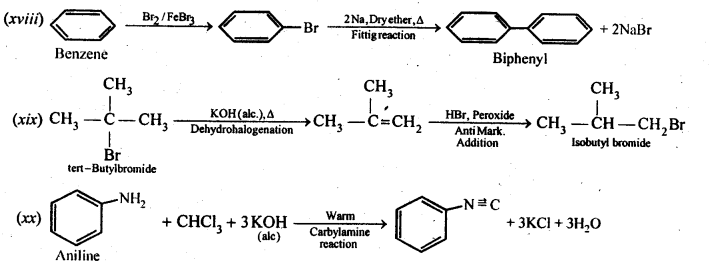
(i)Ethanol to but-l-yne . (ii)Ethane to bromoethene
(iii)Propene to 1-nitropropane (iv)Toluene to benzyl alcohol
(v)Propene to propyne (vi)Ethanol to ethyl fluoride
(vii)Bromomethane to propanone (viii)But-l-ene to but-2-ene
(ix)1-Chlorobutane to n-octane (x)Benzene to biphenyl
Ans.
10.12 Explain why
(i)the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii)alkyl halides, though polar, are immiscible with water?
(iii)Grignard reagents should be prepared under anhydrous conditions?
Ans. (i) sp2-hybrid carbon in chlorobenzene is more electronegative than a sp3-hybrid carbon in cyclohexylchloride, due to greater s-character. Thus, C atom of chlorobenzene has less tendency to release electrons to Cl than carbon atom of cyclohexylchloride.
As a result, C – Cl bond in chlorobenzene is less polar than in cyclohexylchloride. Further, due to delocalization of lone pairs of electrons of the Cl atom over the benzene ring, C-Cl bond in chlorobenzene acquires some double bond character while the C – Cl in cyclohexy! chloride is a pure single bond. In other words, C-Cl bond in chlorobenzene is shorter than in cyclohexyl chloride.
Since dipole moment is a product of charge and distance, therefore, chlorobenzene has lower dipole moment than cyclohexylchloride due to lower magnitude of negative charge on the Cl atom and shorter C-Cl distance.
(ii)Alkyl halides are polar molecules, therefore, their molecules are held together by dipole-dipole attraction. The molecules of H2O are hold together by H-bonds. Since the new forces of attraction between water and alkyl halide molecules are weaker than the forces of attraction already existing between alkyl halide – alkyl halide molecules and water-water molecules, thefefore, alkyl halides are immiscible (not soluble) in water. Alkyl halide are neither able to form H- bonds with water nor are able to break the H-bounding network of water.
(iii)Grignard reagents are very reactive. They react with moisture present in the apparatus to form alkanes
Thus, Grignard reagents must be prepared under anhydrous conditions.
10.13 Give the uses of freon 12, DDT, carbon tetrachloride and iodoform.
Ans. Iodoform: It was earlier used as an antiseptic but the antiseptic properties are due to the liberation of free iodine and not due to iodoform itsdf. Due to its objectionable smell, it has been replaced by other formulations containing iodine.
Carbon tetrachloride:
Uses:
(i)As an industrial solvent for oil, fats, resins etc.and also in dry cleaning.
(ii)CCl4 vapours are highly non inflammable, thus CCl4 is used as a fire extinguisher under the name pyrene.
(iii)Used in the manufacture of refrigerants and propellants for aerosol cans.
Freons: Freon-12 (CCl2F2) is most common freons in industrial use.
Uses: For aerosol propellants, refrigeration and air conditioning purposes.
DDT (p -p’ – Dichloro diphenyl – trichloro ethane):
(i)The use of DDT increased enormously on a world wide basis after World War II, primarily because of its effectiveness against the mosquitoes that spreads malaria and other insects which damages crops..
(ii) However, problems related to extensive use of DDT began to appear in the late 1940 s. Many species of insects developed resistance to DDT, it was also discovered to have a high toxicity towards fishes. DDT is not metabolised very rapidly by animals, instead it is deposited and stored in the fatty tissues. If the ingestion continues at a steady rate, DDT builds up within the animals overtime.
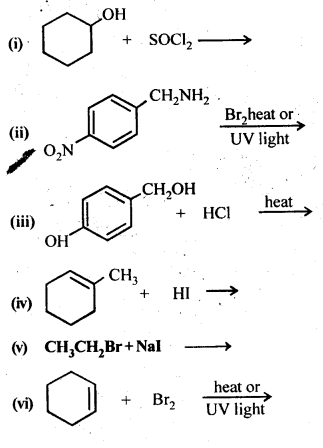
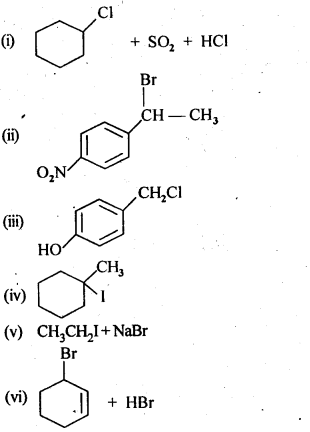
10.14 Write the structure of the major organic product in each of the following reactions:
Ans.
10.15 Write the mechanism of the following reaction:
Ans. KCN is a resonance hybrid of the following two contributing structures:
Thus, CN– ion is an ambident nucleophile. Therefore, it can attack the “carbon atom of C-Br bond in n-BuBr either through C or N. Since C – C bond is stronger than C – N bond, therefore, attack occurs through C to form n-butyl cyanide.
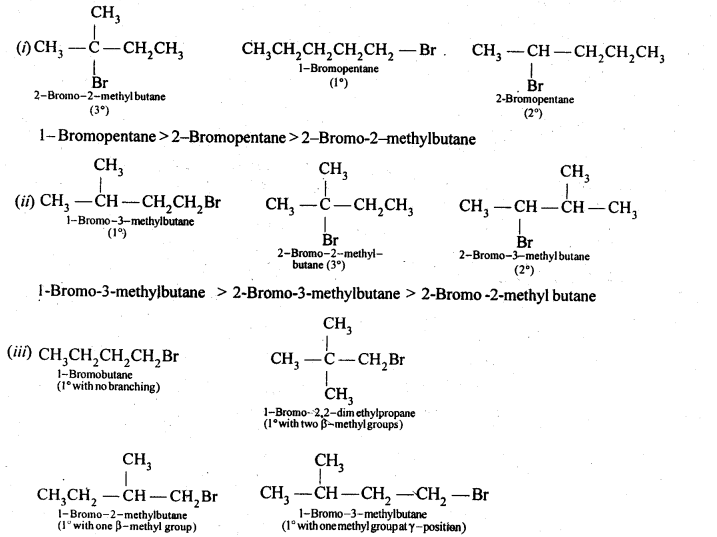
10.16 Arrange the compounds of each set in order of reactivity towards SN2 displacement:
(i) 2-Bromo-2-methyibutane, 1-Bromopentane, 2-Bromopentane.
(ii) l-Bromo-3-methyIbutane, 2-Bromo-2-methylbutane, 3-Bromo-2-methylbutane.
(iii) 1-Bromobutane, l-Bromo-2,2-dimethylpropane, l-Bromo-2-methylbutane, l-Bromo-3-methyl butane.
Ans. The SN2 reactions reactivity depends upon steric hindrance. More the steric hindrance slower the reaction.Thus the order of reactivity will be 1°> 2° >3°
Since in case of 1° alkyl halides steric hindrance increases in the order) n-alkyl halides, alkyl halides with a substituent at any position other than the β-position, one substituent at the β-position, two substituents at the β-position, therefore, the reactivity decreases in the same order. Thus, the reactivity of the given alkyl bromides decreases in the order:
1-Bromobutane > l-Bromo-3-methylbutane > l-Bromo-2-methyjbutane> 1-Bromo-2,2-dimethyl propane.
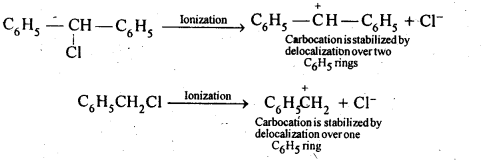
10.17 Out of C6H5CH2Cl and C6H5CHCIC6H5which is more easily hydrolysed by aqueous KOH.
Ans. C6H5CH2Cl is 10 aryl halide while C6H5CH(CI)C6H5 is a 2° aryl halide. In SN1 reactions, the reactivity depends upon the stability of carbocations.
Since the C6H5CHC6H5carbocation is more stable than C6H5CH2 carbocation, therefore,C6H5CHCIC6H5 gets hydrolysed more easily than C6H5CH2Cl under SN1 conditions. However, under SN2 conditions, the reactivity depends on steric hindrance, therefore, under SN2 conditions,C6H5CH2Cl gets hydrolysed more easily than C6H5CHClC6H5.
10.18 p-Dichlorobenzene has higher m.p. and lower solubility than those of o-and m-isomers. Discuss.
Ans. The p-isomer being more symmetrical fits closely in the crystal lattice and thus has stronger inter-
molecular forces of attraction than o- and m-isomers. Since during melting or dissolution, the crystal lattice breaks, therefore, a large amount of energy is needed to melt or dissolve the p-isomer than the corresponding o-and m-isomers. In other words, the melting point of the p-isomer is higher and its solubility lower than the corresponding o-and m-isomers.
10.19 How the following conversions can be carried out:
(i) Propene to propan-l-ol (ii) Ethanol to but-l-yne
(iii) l-Bromopropane to 2-bromopropane (iv) Toluene to benzyl alcohol
(v)Benzene to 4-bromonitrobenzene (vi) Benzyl alcohol to 2-phenylethanoic acid
(vii)Ethanol to propanenitrile (viii) Aniline to chlorobenzene
(ix)2-Chlorobutane to 3,4-dimethylhexane (x) 2-Methyl-1 -propene to 2-chk>ro-2-methylpropane.
(xi)Ethyl chloride to propanoic acid (xii) But-1-ene to n-butyliodide
(xiii)2-Chlropropane to 1-propanol (xiv) Isopropyl alcohol to iodoform
(xv)Chlorobenzene to p-nitrophenol (xvi) 2-Bromopropane to 1-bromopropane
(xvii)Chloroethane to butane , (xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide (xx) Aniline to phenylisocyanide
Ans.
10.20 The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
Ans. If aqueous solution, KOH is almost completely ionized to give OH– ions which being a strong nucleophile brings about a substitution reaction on alkyl halides to form alcohols. Further in the aqueous solution, OH– ions are highly solvated (hydrated). This solvation reduces the basic character of OH– ions which, therefore, fails to abstract a hydrogen from the P-carbon of the alkyl chloride to form alkenes. In contrast, an alcoholic solution of KOH contains alkoxide (RO–) ion which being a much stronger base than OH– ions perferentially eliminates a molecule of HCl from an alkyl chloride to form alkenes.
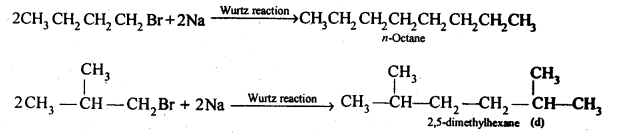
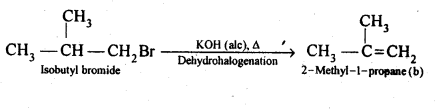
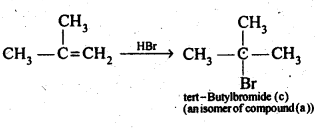
10.21 Primary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b) Compound (b) is reacted, with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it give compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
Ans. (i) There are two primary alkyl halides having the molecular formula, C4H9Br.
(ii) Since compound (a) when reacted with Na metal gave a compound (d) with molecular formula C8H18 which was different from die compound obtained when n-butyl bromide was reacted with Na metal, therefore, (a) must be isobutyl bromide and compound (d) must be 2,3-dimethylhexane.
(iii) If compound (a) is isobutyl bromide, than the compound (b) which it gives on treatment with alcoholic KOH must be 2-methyl-1-propane.
(iv) The compound (b) on treatment with HBr gives compound (c) in accordance with Markownikoff rule. Therefore, compound (c) is tert-butyl bromide which is an isomer of compound (a) ,i.e., isobutyl ‘ bromide.
Thus
(a)is isobutyl bromide,
(b)is 2-methyl-1 -propane,
(c)is tert-butylbromide, and
(d)is 2,5-dimethylhexane.
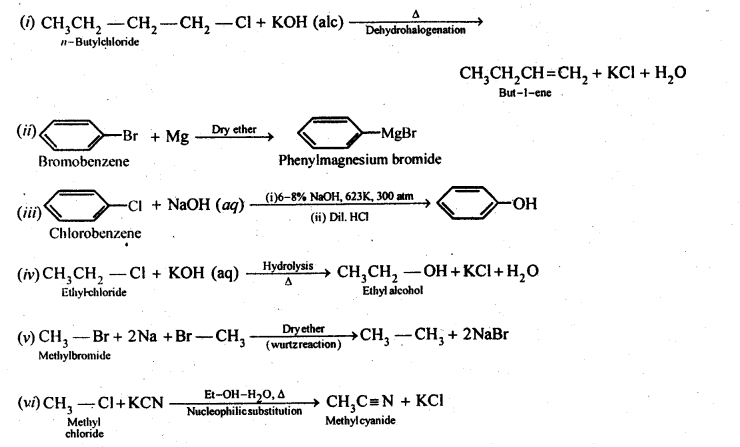
10.22 What happens when .
(i)n-butyi chloride is treated with alcoholic KOH.
(ii)bromobenzene is treated with Mg in the presence of dry ether.
(iii)chlorobenzene is subjected to hydrolysis.
(iv)ethyl chloride is treated with aqueous. KOH.
(v)methyl bromide is treated with sodium in the presence of dry ether,
(vi) methyl chloride is treated with KCN.
Ans.