Page No 160:
Question 1:
What are macromolecules? Give examples.
Answer:
Macromolecules are large complex molecules that occur in a colloidal state in intercellular fluid. They are formed by the polymerization of low molecular weight macromolecules.
Polysaccharides, proteins, and nucleic acids are common examples of macromolecules.
Question 2:
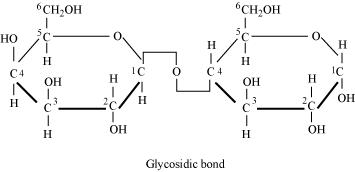
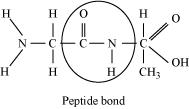
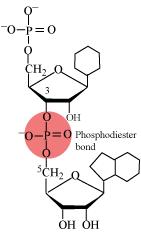
Illustrate a glycosidic, peptide and phospho-diester bond.
Answer:
(a)
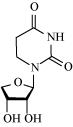
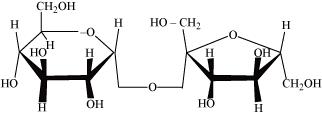
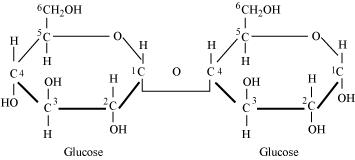
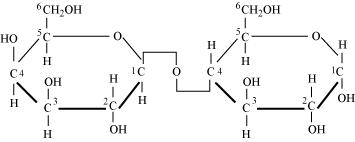
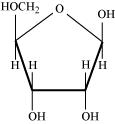
Glycosidic bond is formed normally between carbon atoms, 1 and 4, of neighbouring monosaccharide units.
(b)
Peptide bond is a covalent bond that joins the two amino acids by – NH – CO linkage.
(c)
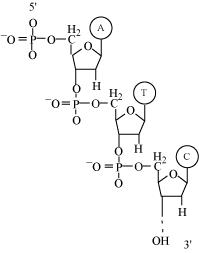
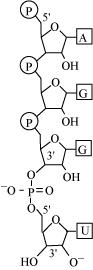
Phosphodiester bond is a strong covalent bond between phosphate and two sugar groups. Such bonds form the sugar phosphate backbone of nucleic acids.
Question 3:
What is meant by tertiary structure of proteins?
Answer:
The helical polypeptide chain undergoes coiling and folding to form a complex three-dimensional shape referred to as tertiary structure of proteins. These coils and folds are arranged to hide the non-polar amino acid chains and to expose the polar side chains. The tertiary structure is held together by the weak bonds formed between various parts of the polypeptide chain.
Question 4:
Find and write down structures of 10 interesting small molecular weight
biomolecules. Find if there is any industry which manufactures the compounds
by isolation. Find out who are the buyers.
Answer:
(a)
| Molecule | Structure | |
| 1. | Adenosine | |
| 2. | Thymidine | |
| 3. | Sucrose | |
| 4. | Maltose | |
| 5. | Lactose | |
| 6. | Ribose | |
| 7. | DNA | |
| 8. | RNA | |
| 9. | Glycerol | |
| 10. | Insulin | |
(b)
| Compound | Manufacturer | Buyer | |
| 1. | Starch products | Kosha Impex (P) Ltd. | Research laboratories, educational institutes, and other industries, which use biomolecules as a precursor for making other products. |
| 2. | Liquid glucose | Marudhar apparels | |
| 3. | Various enzymes such as amylase, protease, cellulase | Map (India) Ltd |
Question 5:
Proteins have primary structure. If you are given a method to know which amino acid is at either of the two termini (ends) of a protein, can you connect this information to purity or homogeneity of a protein?
Answer:
Yes, if we are given a method to know the sequence of proteins, we can connect this information to the purity of a protein. It is known that an accurate sequence of a certain amino acid is very important for the functioning of a protein. If there is any change in the sequence, it would alter its structure, thereby altering the function. If we are provided with a method to know the sequence of an unknown protein, then using this information, we can determine its structure and compare it with any of the known correct protein sequence. Any change in the sequence can be linked to the purity or homogeneity of a protein.
For example, any one change in the sequence of haemoglobin can alter the normal haemoglobin structure to an abnormal structure that can cause sickle cell anaemia.
Question 6:
Find out and make a list of proteins used as therapeutic agents. Find other applications of proteins (e.g., cosmetics, etc.)
Answer:
Proteins used as therapeutic agents are as follows:
1. Thrombin and fibrinogen – They help in blood clotting.
2. Antigen (antibody) – It helps in blood transfusion.
3. Insulin – It helps in maintaining blood glucose level in the body.
4. Renin – It helps in osmoregulation.
Proteins are also commonly used in the manufacture of cosmetics, toxins, and as biological buffers.
Question 7:
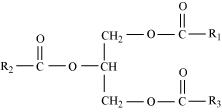
Explain the composition of triglyceride.
Answer:
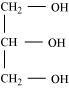
Triglyceride is a glyceride, which is formed from a single molecule of glycerol, esterified with three fatty acids. It is mainly present in vegetable oils and animal fat.
Structure of triglyceride
The general chemical formula of triglyceride is , where R1, R2, and R3 are fatty acids. These three fatty acids can be same or different.
Page No 161:
Question 8:
Can you describe what happens when milk is converted into curd or yoghurt from your understanding of proteins.
Answer:
Proteins are macromolecules formed by the polymerization of amino acids. Structurally, proteins are divided into four levels.
(a) Primary structure – It is the linear sequence of amino acids in a polypeptide chain.
(b) Secondary structure – The polypeptide chain is coiled to form a three-dimensional structure.
(c) Tertiary structure – The helical polypeptide chain is further coiled and folded to form a complex structure.
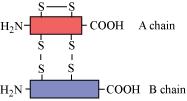
(d) Quaternary structure – More than one polypeptide chains assemble to form the quaternary structure.
Milk has many globular proteins. When milk is converted into curd or yoghurt, these complex proteins get denatured, thus converting globular proteins into fibrous proteins. Therefore, by the process of denaturation, the secondary and tertiary structures of proteins are destroyed.
Question 9:
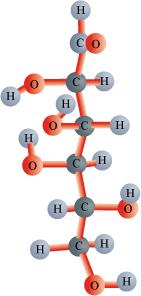
Can you attempt building models of biomolecules using commercially available atomic models (Ball and Stick models).
Answer:
Ball and stick models are 3-D molecular models that can be used to describe the structure of biomolecules.
In ball and stick model, the atoms are represented as balls whereas the bonds that hold the atoms are represented by the sticks. Double and triple bonds are represented by springs that form curved connections between the balls. The size and colour of various atoms are different and are depicted by the relative size of the balls.
It is the most fundamental and common model of representing biomolecular structures.
In the above ball and stick model of D-glucose, the oxygen atoms are represented by red balls, hydrogen atoms by blue balls, while carbon atoms are represented by grey balls.
Question 10:
Attempt titrating an amino acid against a weak base and discover the number of dissociating ( ionizable ) functional groups in the amino acid.
Answer:
Titrating a neutral or basic amino acid against a weak base will dissociate only one functional group, whereas titration between acidic amino acid and a weak acid will dissociate two or more functional groups.
Question 11:
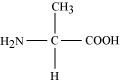
Draw the structure of the amino acid, alanine.
Answer:
Structure of alanine
Question 12:
What are gums made of? Is Fevicol different?
Answer:
Gums are hetero-polysaccharides. They are made from two or more different types of monosaccharides. On the other hand, fevicol is polyvinyl alcohol (PVA) glue. It is not a polysaccharide.
Question 13:
Find out a qualitative test for proteins, fats and oils, amino acids and test any fruit juice, saliva, sweat and urine for them.
Answer:
(a) Test for protein
Biuret’s test – If Biuret’s reagent is added to protein, then the colour of the reagent changes from light blue to purple.
(b) Test for fats and oils
Grease or solubility test
(c) Test for amino acid
Ninhydrin test – If Ninhydrin reagent is added to the solution, then the colourless solution changes to pink, blue, or purple, depending on the amino acid.
| Item | Name of the test | Procedure | Result | Inference | |
| 1. | Fruit juice | Biuret’s test | Fruit juice + Biuret’s reagent | Colour changes from light blue to purple | Protein is present. |
| Grease test | To a brown paper, add a few drops of fruit juice. | No translucent spot | Fats and oils are absent or are in negligible amounts. | ||
| Ninhydrin test | Fruit juice + Ninhydrin reagent + boil for 5 minutes | Colourless solution changes to pink, blue, or purple colour | Amino acids are present. | ||
| 2. | Saliva | Biuret’s test | Saliva + Biuret’s reagent | Colour changes from light blue to purple | Proteins are present. |
| Grease test | On a brown paper, add a drop of saliva. | No translucent spot | Fats/oils are absent. | ||
| Ninhydrin test | Saliva + Ninhydrin reagent + boil for 5 minutes | Colourless solution changes to pink, blue, or purple colour | Amino acids are present. | ||
| 3. | Sweat | Biuret’s test | Sweat + Biuret’s reagent | No colour change | Proteins are absent. |
| Solubility test | Sweat + Water | Oily appearance | Fats/oil may be present. | ||
| Ninhydrin test | Sweat + Ninhydrin reagent + boil for 5 minutes | No colour change, solution remains colourless | Amino acids are absent. | ||
| 4. | Urine | Biuret’s test | Few drops of urine + Biuret’s reagent | Colour changes from light blue to purple | Proteins are present. |
| Solubility test | Few drops of urine + Water | Little bit of oily appearance | Fats may or may not be present. | ||
| Ninhydrin test | Few drops of urine + Ninhydrin reagent + boil for 5 minutes | Colourless solution changes to pink, blue, or purple colour depending on the type of amino acid | Amino acids are present. | ||
Question 14:
Find out how much cellulose is made by all the plants in the biosphere and compare it with how much of paper is manufactured by man and hence what is the consumption of plant material by man annually. What a loss of vegetation!
Answer:
Approximately, 100 billion tonnes of cellulose are made per year by all the plants in the biosphere and it takes 17 full grown trees to make one ton of paper. Trees are also used to fulfil the other requirements of man such as for timber, food, medicines, etc. Hence, it is difficult to calculate the annual consumption of plant material by man.
Question 15:
Describe the important properties of enzymes.
Answer:
Properties of enzymes
(1) Enzymes are complex macromolecules with high molecular weight.
(2) They catalyze biochemical reactions in a cell. They help in the breakdown of large molecules into smaller molecules or bring together two smaller molecules to form a larger molecule.
(3) Enzymes do not start a reaction. However, they help in accelerating it.
(4) Enzymes affect the rate of biochemical reaction and not the direction.
(5) Most of the enzymes have high turnover number. Turnover number of an enzyme is the number of molecules of a substance that is acted upon by an enzyme per minute. High turnover number of enzymes increases the efficiency of reaction.
(6) Enzymes are specific in action.
(7) Enzymatic activity decreases with increase in temperature.
(8) They show maximum activity at an optimum pH of 6 – 8.
(9) The velocity of enzyme increases with increase in substrate concentration and then, ultimately reaches maximum velocity.